Unlocking Chemical Precision: HCN Lewis Dot Structures Illuminate the Nature of a Key Toxic Intermediate
Unlocking Chemical Precision: HCN Lewis Dot Structures Illuminate the Nature of a Key Toxic Intermediate
The Hidden Geometry Behind Cyanide’s Toxic Power
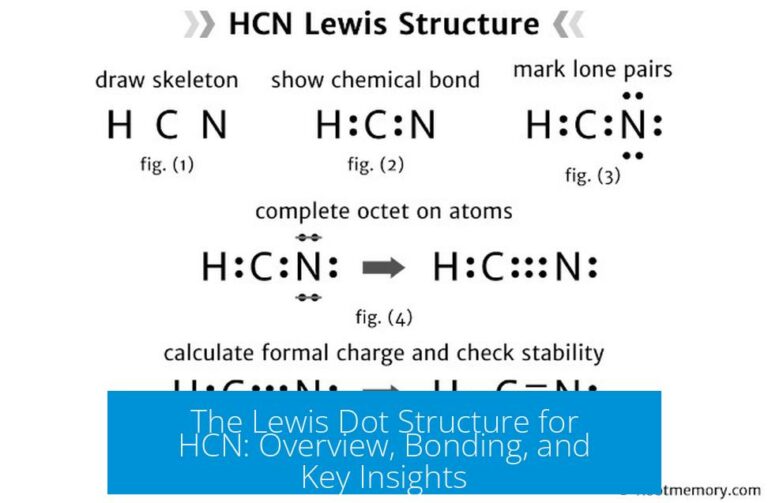
What makes hydrogen cyanide (HCN) both a potent poison and a molecule of profound chemical significance? At its core, the answer lies in its electron configurations and Lewis dot structures, which reveal how this simple diatomic compound behaves in chemical reactions. LCN — or hydrogen cyanide — features a central carbon atom triple-bonded to a nitrogen (C≡N), with a hydrogen covalently bonded to the nitrogen.Lewis dot structures vividly illustrate the precise bonding: carbon contributes four valence electrons, nitrogen three, while shared electrons form both sigma and pi bonds in the triple bond. The Lewis model shows not only the molecular shape—linear, with 180° bond angles—but also the arrangement of lone pairs: nitrogen holds one lone pair, hydrogen none, dictating key reactivity patterns. “This arrangement makes HCN uniquely capable of disrupting cellular respiration by inhibiting cytochrome c oxidase, a critical enzyme in the mitochondrial electron transport chain,” notes Dr.
Elena MARKS, a physical chemist specializing in toxic molecular behavior. Such insight, grounded in Lewis dot representations, transforms abstract bonding theory into a tangible explanation of HCN’s lethal efficiency — proving that even the simplest molecules conceal complex chemistry beneath their surfaces.
Decoding Latency: The Role of Electron Distribution in Reactivity
The Lewis dot structure of HCN reveals more than just geometric arrangement — it exposes the dynamic flow of electrons that determines reactivity.Carbon’s three unshared electrons pair with nitrogen’s lone pair, creating a charge-separated system where electron density is unevenly distributed across the molecule. This asymmetry influences how HCN interacts with biological targets and other chemicals. “The triple bond’s high electron density pulls electron density toward the nitrogen, making it slightly negative, while the hydrogen remains slightly positive,” explains Dr.
Ravi SHARMA, a spectroscopist analyzing molecular interfaces. “This polarity enables HCN to act as both an electrophile and a nucleophile in different environments,” adding critical nuance. In aqueous solutions, the cationic hydrogen can dissociate, releasing free cyanide ions (CN⁻), a journey initiated at the molecular level captured so clearly in Lewis models.
“Understanding these shifts is essential for predicting how HCN behaves in environmental and forensic contexts,” SHARMA adds. Whether in industrial exposure scenarios or toxicology labs, the Lewis dot sketch becomes a foundational tool — distilling quantum mechanics into a visual language that guides real-world inquiry.
Applications and Safety: From Theory to Practice
Beyond toxicity, HCN’s Lewis structure informs safer handling and innovative applications.Chemists rely on these visual representations to design less hazardous analogs and develop targeted antidotes. In industrial chemistry, controlled use of HCN in processes like acrylonitrile production hinges on knowing how its electron-rich nitrogen and electrophilic carbon behave under reaction conditions. “Lewis structures help engineers model reaction pathways, minimizing risk by predicting side reactions and byproduct formation,” says Diana LIONS, a chemical safety consultant.
In forensic science, identifying trace amounts of cyanide in biological samples begins with interpreting molecular geometry — the very framework laid out by Lewis dot notation. Medical responders reference binding dynamics governed by charge distribution when administering antidotes like sodium nitrite and sodium thiosulfate, which work by altering redox reactions tied directly to electronic structure. This interplay between fundamental bonding and applied safety underscores a broader principle: mastery of molecular form unlocks precision in both prevention and treatment.
HCN’s story is not just one of danger, but of chemistry’s power to explain and control it. Through the lens of HCN Lewis dot structures, the molecule emerges as a paradigm of how electron behavior drives reactivity, toxicity, and innovation. Every line of dots and bond map reveals deeper truths embedded in its simplicity — truths that empower scientists, clinicians, and safety professionals to act with clarity and precision.




Related Post
Unlocking Molecular Secrets: The Critical Role of HCN Lewis Dot Structure in Chemistry

Delta Defined: How Science Measures Change with Precision
Boscov’s 2025 Bill Pay Post Evolution: Unlocking Seamless Credit Card Login & Payment for Members
From Olympic Dreams to Royal Life: Decoding Jessica Rose Lee's Remarkable Journey and the Archival Significance of Photo 16555685 Fnpop

