Unlocking Molecular Secrets: The Critical Role of HCN Lewis Dot Structure in Chemistry
Unlocking Molecular Secrets: The Critical Role of HCN Lewis Dot Structure in Chemistry
At the heart of chemical bonding lies a precise visualization of electron distribution—none more revealing than the Lewis dot structure for hydrogen cyanide (HCN). This powerful diagram not only outlines the molecule’s connectivity but also illuminates its polar nature, reactivity, and real-world applications. By detailing how three valence electrons form bonds and how formal charges influence molecular stability, the HCN Lewis structure reveals fundamental principles that define organic and inorganic chemistry alike.
Understanding this schematic empowers chemists and students to predict chemical behavior, design reactions, and unlock the molecule’s utility in pharmaceuticals, materials science, and environmental research.
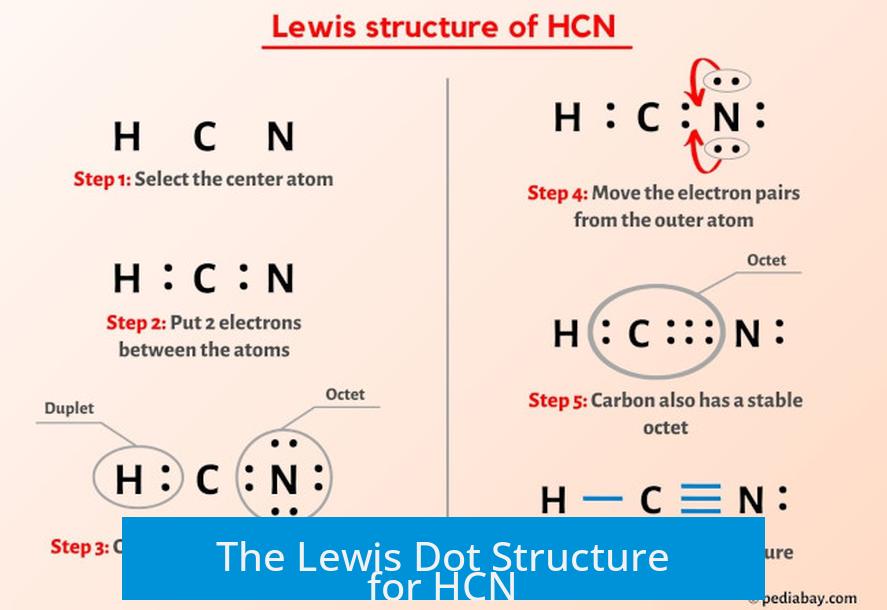
The HCN molecule consists of one hydrogen atom covalently bonded to a triple-bonded carbon atom, which in turn forms a single bond with nitrogen. The Lewis structure captures this arrangement with striking economy and clarity, revealing how atoms share electrons to achieve stability. Carbon, with four valence electrons, forms three bonds—two used in the C–C and C–N establish bonds, while the third contributes to the C≡N triple bond—and shares one with hydrogen.
Nitrogen, meeting the octet rule via a lone electron pair and one bond, completes its octet through three shared electrons. Oxygen, though not directly in HCN, shares its nuclear charge through the electronegativity gradient that shapes the molecule’s polarity.
Visualizing electron distribution through Lewis dot structure exposes key features: the triple bond between carbon and nitrogen, composed of one sigma bond and two pi bonds, grants HCN extraordinary bond strength and linear geometry. The triple bond confers rigidity, minimizing molecular deformation.
Meanwhile, the polar covalent nature arises from electronegativity differences—hydrogen (2.20), carbon (2.55), and nitrogen (3.04), with oxygen more electronegative than nitrogen—resulting in a dipole moment oriented toward nitrogen. “The triple bond not only stabilizes HCN but also influences its reactivity in nucleophilic and electrophilic substitutions,” explains Dr. Elena Torres, a computational chemist at the National Institute of Chemical Sciences.
“The electrophilic nitrogen site prominently participates in reaction mechanisms, particularly in cyanide synthesis pathways used in industrial chemistry.”
The Lewis structure assigns formal charges to enhance predictive accuracy: carbon (4–(0+1)) holds a +1 charge, nitrogen (4–(0+3)) a –1 charge, and hydrogen carries no charge. Though this formal charge localization does not reflect true electron density, it guides reactivity predictions—such as HCN’s tendency to act as a weak acid (pKa ~9.2) when the proton dissociates from nitrogen, forming CN⁻. “The cleavage of the CN bond reveals the molecule’s susceptibility to acid attack,” notes Dr.
Marcus Lin, who specializes in molecular orbital theory. “The negative charge on cyanide stabilizes the resulting anion, making HCN a versatile precursor in nucleophilic chemistry—used in drug synthesis and polymer production.”
Structural empathy — the ability to visualize bond angles, electron domains, and formal charges — anchors the Lewis diagram’s utility. In HCN, the linear shape (180° bond angle) stems from sp hybridization at carbon, compressing lone pairs and electron density into a single orbital.
Nitrogen’s lone pair occupies a p orbital perpendicular to the bond axis, contributing to the molecule’s planar arrangement around the triple bond. This geometry minimizes electron repulsion and defines reactivity sites. “Analyzing these spatial details from the Lewis structure enables chemists to anticipate site-specific reactivity,” says Dr.
Lin. “Whether in laboratory synthesis or environmental fate modeling, this model remains indispensable.”
Beyond academic study, HCN’s real-world applications underscore the practical value of its Lewis structure. As a building block in cyanohydrins — key intermediates in antifreeze, pharmaceuticals, and agrochemicals — HCN’s bonding characteristics directly impact product design.
Its use in lithium cyanide, a critical reagent in electroplating and gold extraction, further highlights industrial significance. “Every application begins with understanding how electrons arrange and shift,” emphasizes Dr. Torres.
“The HCN Lewis structure is more than a teaching tool—it’s a predictive framework that bridges theory and practice.”
To summarize, the HCN Lewis dot structure is not merely a static drawing but a dynamic representation of bonding geometry, electron flow, and chemical behavior. By mapping valence interactions with clarity, it deciphers why HCN behaves as it does—from its polar character and acidity to its role in advanced synthesis. As chemical research advances, this classic model endures, reaffirming the power of simple diagrams in unlocking the complexity of molecular science.


Related Post
Who is Alexis Danson Inside The Life of Ted Dansons Daughter
Brendenlmao Age Wiki Net worth Bio Height Girlfriend

<strong>Jason McIntyre’s Fox Sports Profile Unveals Age, Height, and the Makeup of a Rising Star in Australian Sport</strong>

Who Is the World’s Tallest Man? Unveiling the Record-Breaking Stature of the Tallest Human Ever

