Unlocking Chemical Mysteries: The Precision and Power of N Lewis Dot Structure
Unlocking Chemical Mysteries: The Precision and Power of N Lewis Dot Structure
At the heart of molecular chemistry lies a deceptively simple yet profoundly insightful tool: the Lewis dot structure, particularly for nitrogen-containing compounds. These diagrams, built on Gilbert N. Lewis’s foundational model of electron sharing, reveal how atoms bond and arrange themselves in space.
By mapping valence electrons and bonding patterns, N Lewis Dot Structures transform abstract quantum behavior into a visual language scientists use daily to predict reactivity, stability, and molecular geometry. This structure is not just a classroom illustration—it’s a cornerstone of modern chemistry, guiding research in materials science, pharmacology, and catalysis.
Why Nitrogen Matters in Lewis Dot Diagrams
Nitrogen occupies a unique and critical role in organic and inorganic chemistry, and its Lewis dot structure illuminates why. With five valence electrons—three unpaired and one pair—nitrogen engages in diverse bonding chemistries.
Its ability to form single, double, or triple bonds thanks to lone pairs and available orbitals makes it indispensable in proteins, nucleic acids, and energy storage molecules. “Nitrogen’s electron configuration gives it a chemical personality defined by both inertness and reactivity,” notes Dr. Elena Torres, a chemical educator.
“Lewis structures bring this duality into sharp relief.”
In molecular terms, nitrogen typically forms three sigma bonds using three lone pairs orbitals, or two sigma and one pi bond when participating in double or triple bonds. This flexibility stems from nitrogen’s sp³ hybridization in compounds like ammonia (NH₃), where a lone pair resides in an unhybridized p orbital, creating a trigonal pyramidal geometry. The precise placement of electrons reveals not just connectivity but molecular shape—key to understanding function.
Building the Nitrogen Dot Structure: Step-by-Step
To construct an N Lewis Dot Structure, begin with the atomic symbol nitrogen (N), a nonmetal in group 15 with five valence electrons.
Follow this structured process:
- Start by placing the N atom at the center, as it forms bonds and often carries a partial positive charge in stable molecules like NO₂.
- Attach the five valence electrons as individual dots around the N: three to form unpaired electrons or initial bonds, and two to create a lone pair.
- Use single lines (bonds) to connect N to bonded atoms—each bond represents a shared pair of electrons.
- Fill remaining electron




Related Post

Unlocking Atomic Secrets: The Essential Boron Lewis Dot Structure

Decode the Invisible: How Nitrogen’s Lewis Dot Structure Illuminates Key Chemical Behavior
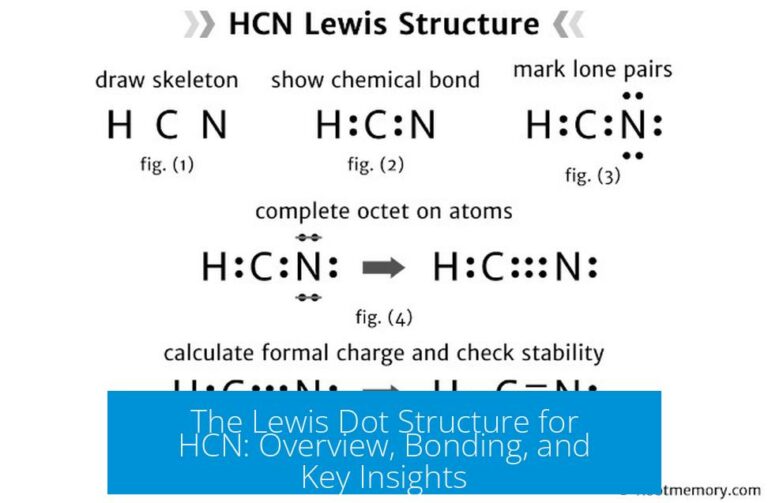
Unlocking Molecular Secrets: The Critical Role of HCN Lewis Dot Structure in Chemistry
WWE Nixes Longtime Production Rule Limiting Superstar Attire

