How Many Protons Defines Hydrogen? The Key to All Creation
How Many Protons Defines Hydrogen? The Key to All Creation
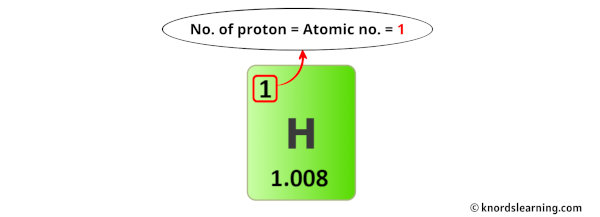
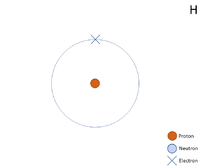
Hydrogen, the simplest and most abundant element in the universe, is defined at its core by a single proton in its atomic nucleus—just one, but only one. This singular proton is the foundation of hydrogen’s identity, dictating its chemical behavior, its role in stellar fusion, and its prevalence across galaxies. With a proton count of one, hydrogen stands apart not only as the lightest of all elements but as the building block of matter itself.
Hydrogen contains exactly one proton in its nucleus, a fact that shapes nearly every aspect of its physical and chemical behavior.As the first element on the periodic table, hydrogen sits at the far left: atomic number 1, meaning one proton defines its elemental identity. This simplicity gives hydrogen extraordinary influence—despite its minute atomic size, hydrogen atoms form water, methane, ammonia, and countless organic molecules essential to life.
Each hydrogen atom’s nucleus, composed solely of one proton, determines how it interacts with other elements and energy. Unlike heavier atoms, hydrogen’s simplicity allows fusion reactions in stars to proceed efficiently, powering the sun and enabling the formation of heavier elements.
The proton in hydrogen’s nucleus is critical to its role in nuclear fusion.In the Sun, hydrogen nuclei combine under extreme pressure and temperature, fusing to form helium and releasing vast amounts of energy—an ongoing process that sustains stellar life cycles. “One proton initiates a cascade of transformations that light stars and shape galaxies,” notes Dr. Elena Mikhailov, a nuclear physicist at the Max Planck Institute.
“It’s a humble particle with outsized power.” This single proton also explains hydrogen’s unique position in atomic structure. With only one proton and typically one electron (in neutral atoms), hydrogen exhibits versatile bonding patterns—ranging from simple covalent bonds in water (H₂O) to electressional interactions in metallic hydrogen and plasma states in interstellar space. Unlike multi-proton atoms such as carbon or oxygen, hydrogen’s nuclear simplicity enables rapid reaction kinetics, essential in chemical equilibrium and biological systems.
In the realm of matter, isotopes of hydrogen—protium, deuterium, and tritium—differ only by the number of neutrons, but the core proton count remains unchanged, preserving hydrogen’s defining characteristic. Protium, the most common isotope, contains no neutrons; deuterium includes one, and tritium carries two. Yet none escape the elemental signature set by the lone proton.
The significance of hydrogen’s single proton extends beyond atomic theory into practical applications.In fuel cells, hydrogen’s proton conductance enables clean energy conversion. In astrophysics, hydrogen’s fusion dynamics govern stellar evolution and cosmic structure. Moreover, hydrogen’s simplicity makes it a model system for studying quantum mechanics and nuclear physics—offering insight into the forces binding matter at its most fundamental level.
In essence, how many protons does hydrogen have? The answer—one—anchors one of nature’s most consequential elements. It reveals a truth at the heart of matter: complexity often arises from simplicity.
The one proton within hydrogen’s nucleus is not just a particle; it is the origin of hydrogen’s power, its ubiquity, and its pivotal role across the cosmos—from the dawn of stars to the chemistry of life.

Related Post
Tom Sandoval Movies Bio Wiki Age Ariana Madix Restaurant and Net Worth

Unlocking Pikin Meaning: The Hidden Language Shaping Understanding in Today’s World

Best Goalkeeper World Cup 2018: Who Took The Golden Glove?

