Magnesium Ion Formation: Unlocking Electron Loss at the Atomic Level
Magnesium Ion Formation: Unlocking Electron Loss at the Atomic Level
When magnesium atoms engage in chemical transformations, they frequently shed electrons to achieve greater stability—a process exemplified by magnesium ion formation. At its core, this phenomenon reveals the delicate balance of electron dynamics that govern elemental reactivity, with profound implications across industrial, biological, and material sciences. Understanding how magnesium loses electrons not only clarifies fundamental chemistry but also unlocks applications ranging from battery technology to human health.
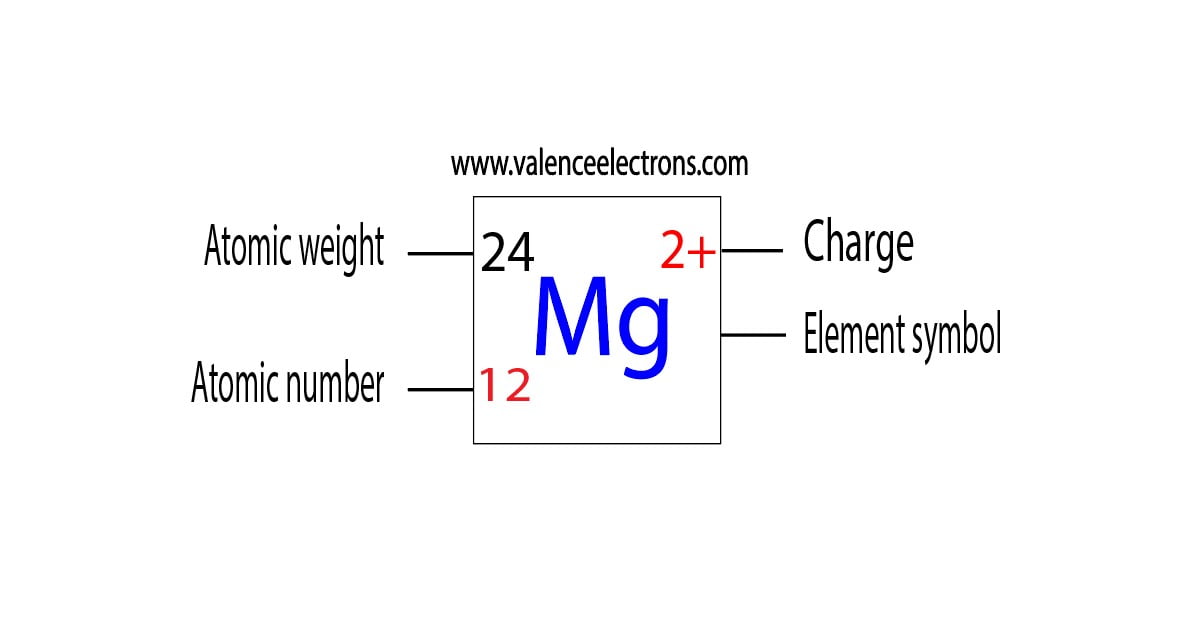
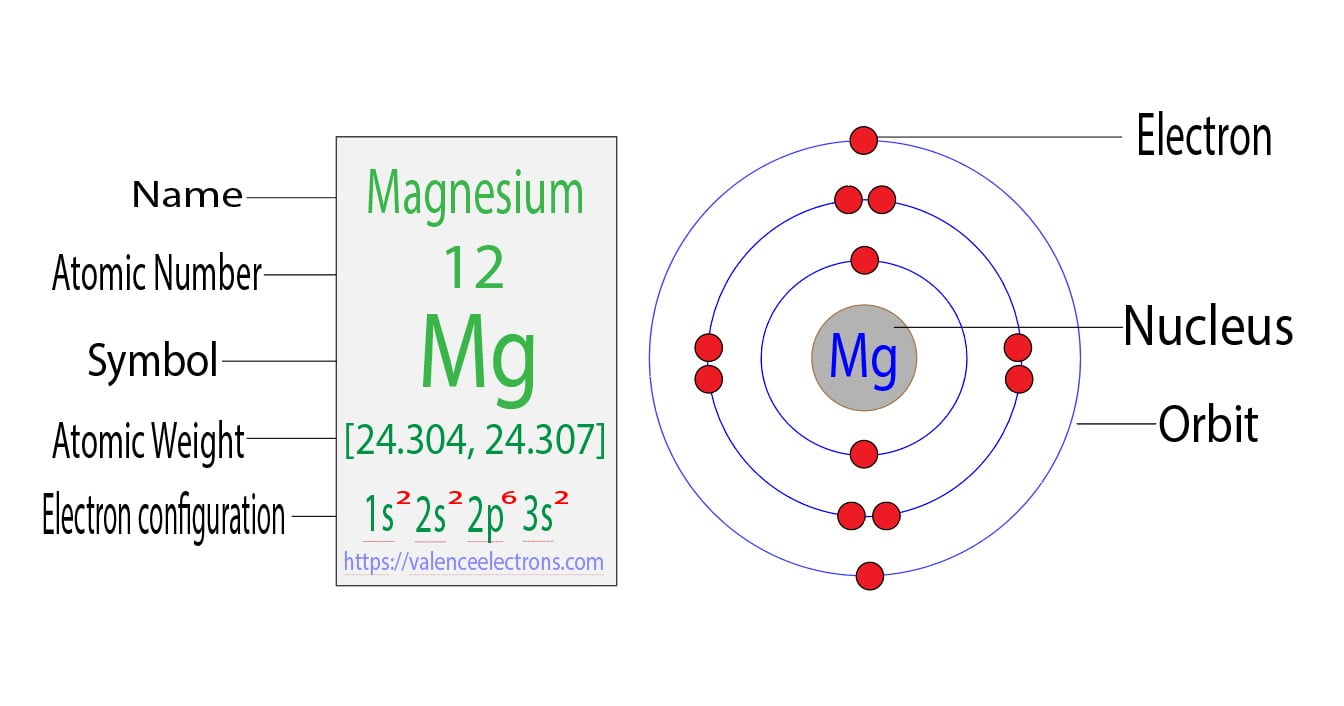
Magnesium ions—formed when magnesium atoms lose electrons—serve as critical players in countless reaction mechanisms, transforming passive ions into active participants in electrochemical and enzymatic pathways. The formation of magnesium ions begins with ionization, the deliberate removal of valence electrons from neutral magnesium atoms. With two electrons in its outermost shell (forcing configuration 3s²), magnesium readily relinquishes these tightly held electrons under specific conditions.
Unlike metals that share electrons broadly, magnesium’s ionic behavior is highly predictable: each loss elevates its charge state gradually. When magnesium loses one electron, it becomes Mg²⁺—a monovalent cation whose charge is stabilized by octet electron configurations in neighboring species.
The Electron Loss Process: From Atom to Ion
Electron loss in magnesium follows a stepwise, energy-dependent pathway governed by environmental factors such as pH, temperature, and ionic medium.The first ionization energy—the energy required to remove the first electron—measures magnesium’s inherent tendency to donate electrons. With an initial ionization energy of approximately 738 kilojoules per mole, magnesium ranks in the mid-table among metals, reflecting its solid metallic character rather than being exceptionally active or inert. Once the first electron departs, the resulting Mg⁺ ion remains loosely bound, though increasingly unstable.
The second ionization phase proves significantly more demanding, requiring 1450.7 kJ/mol to remove a second electron. This substantial jump arises because removing an electron from a positively charged ion strengthens nuclear-electron attraction, making further ejections energetically prohibitive without intense conditions. As a result, magnesium ion formation is often a controlled, deliberate process—rarely spontaneous outside high-energy environments like concentrated acid solutions or electrolytic cells.
Key Factors Influencing Electron Removal: - **Electron Shielding and Nuclear Charge:** Extra protons in magnesium’s nucleus exert stronger pull on electrons, increasing ionization thresholds. - External Conditions: Acidic media protonate magnesium species, enhancing electron loss efficiency by stabilizing cations. - **Coordination Effects: In aqueous solutions, hydration shells surround emerging ions, altering effective energetics.
- **Electronic Configuration Stability: The loss of two electrons transforms magnesium into a fully positively charged ion, diminishing repulsion but amplifying sensitivity to surrounding ions.
Beyond theoretical interest, magnesium ion formation underpins real-world utility. In alkaline battery electrolytes, for example, magnesium ions facilitate ion transport between electrodes, directly impacting energy storage efficiency.
Similarly, cellular magnesium regulation—where Mg²⁺ ions act as essential cofactors in enzyme activation—depends on precise ion release mechanisms tightly governed by electron transfer dynamics.
Applications and Implications in Modern Science
Magnesium’s predictable electron loss enables its use in high-performance materials. Magnesium-based alloys, prized for lightweight strength, rely on controlled ion release during manufacturing and service. In biomedicine, magnesium supplements leverage the ion’s bioavailability—small amounts of Mg²⁺ are critical for neurotransmission, muscle contraction, and enzymatic function—yet precise dosage is essential to avoid toxicity, a balance dictated by electron availability and cellular kinetics.Moreover, magnesium ion formation informs green chemistry efforts. In sustainable battery research, magnesium’s divalent nature promises higher energy density than lithium-ion systems, but mastering its electron withdrawal remains a key engineering challenge. Electrochemical studies further reveal that Mg²⁺ mobility in solid electrolytes hinges not only on ion concentration but on electron identity and circuit pathways, underscoring its role as both a participant and regulator in redox reactions.
While magnesium ions may appear simple in structure, their formation is a nuanced dance of energy, environment, and electron behavior. Mastery of this process empowers innovations across energy storage, materials engineering, and medicine—inviting continued exploration into how electron loss shapes elemental destiny at the atomic scale.


Related Post
Mark Langston KLAAFM Bio Wiki Age Height Family Wife Illness Baseball Salary And Net Worth
Unpacking the Complex World of White Collar Actions: Key Insights into John Bolz's Ethical Framework

Find My iPhone Quick Guide: Master the Art of Locating Your Lost Device in Seconds

