X-Linked Hyper IgM Syndrome (HIGM1): The Rare Immune Defect That Hides in Plain Sight
X-Linked Hyper IgM Syndrome (HIGM1): The Rare Immune Defect That Hides in Plain Sight
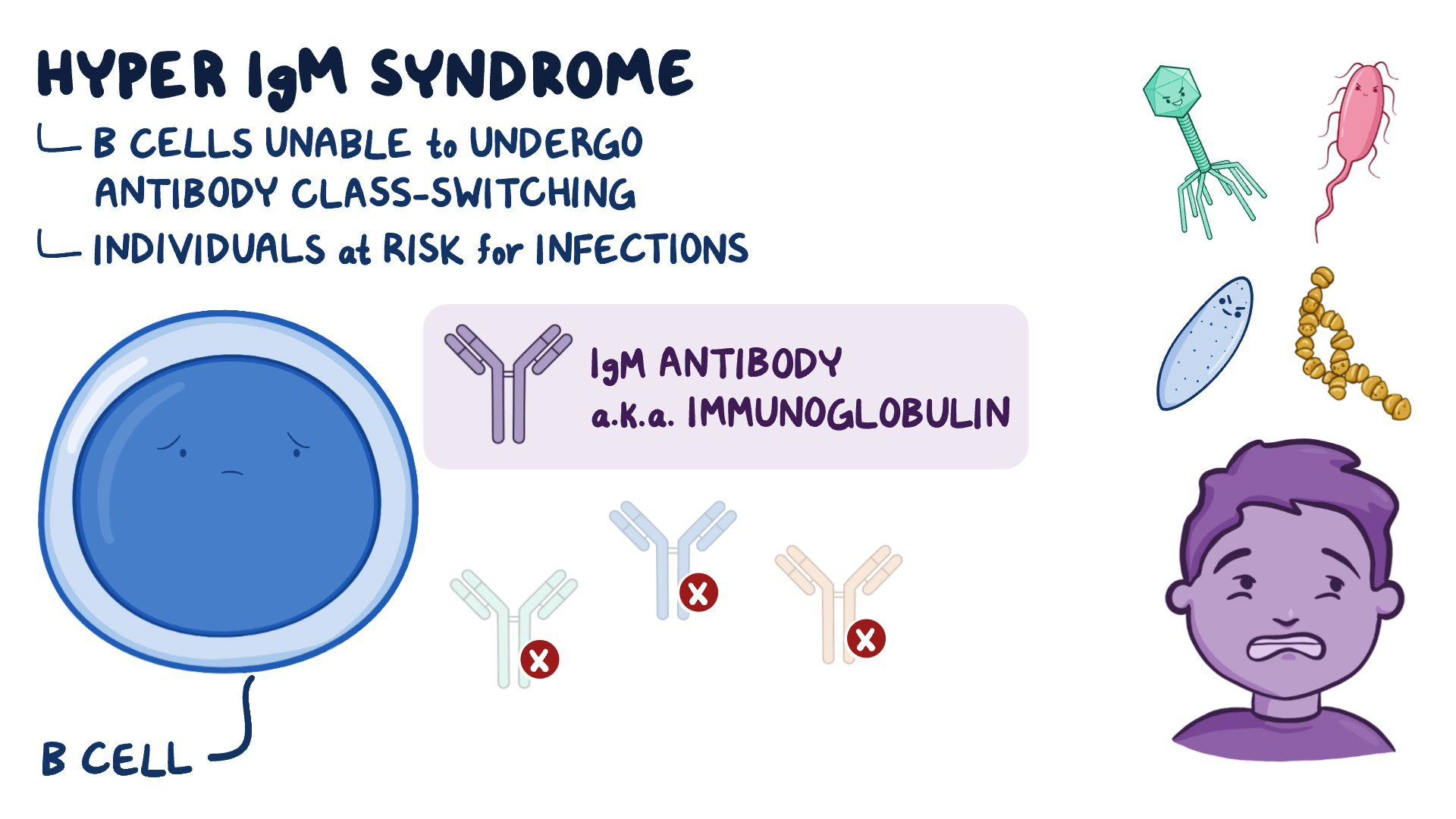
X-Linked Hyper IgM Syndrome (HIGM1) is a rare genetic disorder characterized by a profound defect in immunoglobulin synthesis, leaving patients shockingly vulnerable to recurrent infections. Unlike more common immunodeficiencies, HIGM1 stems from mutations in a single gene—*HIGM1* (also known as *IGHM*)—that disrupts B-cell development and antibody production, particularly impairing the switch from IgM to other antibody classes. This genetic nuance translates into a clinical puzzle: patients present with hallmarks of immune failure but remain genetically distinct, often underdiagnosed for years.
Understanding HIGM1 demands careful attention to its molecular origins, the subtle yet severe symptoms it triggers, and the evolving therapeutic landscape that offers new hope.
Rooted in a single gene mutation, X-Linked Hyper IgM Syndrome (HIGM1) emerges from inheriting a defective *IGHM* gene on the X chromosome, affecting roughly 1 in every 200,000 individuals worldwide. The *IGHM* gene encodes the membrane-bound immunoglobulin μ (overline), a critical component in B-cell receptor signaling and the process by which B cells switch from producing IgM to shaping downstream IgA and IgG responses.
When functional, this genetic blueprint enables the maturation of mature B cells capable of diverse antibody production. But in HIGM1, a mutation disrupts this pathway, leaving patients trapped in a state of nearly exclusive IgM with lesser or absent levels of downstream immunoglobulins IgA and IgG. “This defect isn’t just a numbers game—it’s a systemic failure of adaptive immunity,” explains Dr.
Elena Torres, immunogeneticist at the National Institutes of Health. “Without functional IgA and IgG, the body’s defenses against bacteria and viruses are drastically weakened, resulting in chronic, often lethal infections in early childhood.”
Understanding the molecular basis sets the stage for recognizing the hallmark symptoms, which typically emerge in infancy or early childhood. Children with HIGM1 are paradoxically characterized by elevated IgM levels—sometimes among the highest seen in any human condition—despite an apparent immune stagnation.
Their clinical picture includes:
- Recurrent Severe Infections: Often involving the respiratory tract, ears, and sinuses, with common pathogens including *Streptococcus pneumoniae*, *Haemophilus influenzae*, and *Pneumocystis jirovecii*. These infections are severe, frequent, and poorly responsive to standard antibiotic therapies.
- Autoimmune Manifestations: Paradoxically, despite immunodeficiency, patients may develop autoimmune conditions such as hemolytic anemia or thrombocytopenia, likely due to dysregulated B-cell activity and loss of immunological tolerance.
- Chronic Diarrhea and Malabsorption: GI involvement from mucosal inflammation underscores impaired immune surveillance in the gut, impairing nutrient absorption and growth in young patients.
- Immunodeficiency-Related Complications: Opportunistic infections, mucocutaneous candidiasis, and persistence of opportunistic worms like *Strongyloides* are frequent and life-threatening without timely intervention.
Diagnosing HIGM1 remains a clinical challenge due to its rarity and symptom variability. The cornerstone of diagnosis lies in identifying markedly elevated immunoglobulin M (IgM) levels—often >100 mg/dL—combined with severely reduced or undetectable levels of IgG and IgA, along with normal or near-normal CD19+ B-cell counts.
Normal B-gene excision events on flow cytometry confirm impaired class-switch recombination, pointing directly to *IGHM* dysfunction. Advances in genetic testing now allow definitive diagnosis through targeted sequencing of the *IGHM* gene, enabling precise confirmation even in ambiguous cases.
Early diagnosis is critical, given the rapid progression of uncontrolled infections.
“A delay in recognition can mean the difference between survival and catastrophic illness,” warns Dr. Torres. “Because HIGM1 mimics other immunodeficiencies, heightened clinical suspicion—especially in infants with recurrent sinopulmonary infections combined with elevated IgM—is essential.”
Treatment for HIGM1 centers on restoring immunological balance through immunoglobulin replacement therapy (IRT), yet no single approach fully corrects the underlying defect.
The mainstays include:
- Regular Intravenous or Subcutaneous Immunoglobulin Administration: This life-sustaining therapy replaces missing IgG and IgA, dramatically reducing infection frequency and severity. Administered every 3–4 weeks, IRT typically halts recurrent pneumonia, sepsis, and GI complications, turning what was once a fatal chronic condition into a manageable one.
- Prophylactic Antibiotics and Antifungals: Daily or periodic use of antibiotics and antifungal agents is often necessary to forestall invasive infections during particularly vulnerable periods, such as infancy and early childhood.
- Bone Marrow Transplantation (BMT): The only potentially curative option, BMT replaces the defective hematopoietic stem cell population with healthy donor-derived cells capable of normal immunoglobulin synthesis. Success depends on rigorous donor matching—specifically HLA-identical siblings—and timing, ideally before the first severe infection.
“While high-risk, early BMT offers a chance at lifelong immunological recovery,” notes Dr. James Li, pediatric immunologist at Boston Children’s Hospital.
Emerging research expands the horizon for HIGM1 patients. Gene therapy strategies targeting correction of the *IGHM* mutation are in preclinical development, aiming to restore functional B-cell maturation at its genetic source.
Additionally, personalized IRT regimens tailored to individual antibody profiles are being refined to better mimic natural immune substance dynamics.
The Delicate Balance: Symptoms That Define the HIGM1 Profile
The symptoms of X-Linked Hyper IgM Syndrome reflect a core paradox: abundant IgM with limited adaptive defense. Elevated IgM levels—often the highest observed in any human disorder—serve as both a diagnostic hallmark and a window into immune dysregulation.This imbalance manifests in a cascade of clinical realities:
- **Chronic Infections:** With deficient IgG and IgA, especially vulnerable to bacterial pneumococcus, encapsulated streptococci, and opportunistic organisms like *Pneumocystis jirovecii*. These infections often present with delayed diagnosis, high hospitalization rates, and prolonged recovery.
- **Autoimmune Dysregulation:** Though immunocompromised, the loss of regulatory functions leads to autoantibody production, manifesting as hemolytic anemia, thrombocytopenia, or inflammatory arthritis—complicating disease management.
- **Gastrointestinal Manifestations:** Mucosal immune failure results in chronic diarrhea, malabsorption, and failure to thrive, particularly evident in undiagnosed infants.
- **Persistent Immune Activation:** Despite low functional antibodies, patients endure constant immune system activation, with recurring inflammation contributing to autoimmune complications and systemic strain.
These multifaceted symptoms underscore why early, accurate diagnosis is not merely medical urgency but a lifeline—enabling timely immunoglobulin replacement or bone marrow transplantation before irreversible organ damage occurs.
For families navigating X-Linked Hyper IgM Syndrome, the journey is defined by resilience, precision medicine, and a deepening scientific understanding.
From diagnosing elevated IgM in a newborn to managing lifelong IRT or planning curative BMT, each step bridges cutting-edge immunotherapy with compassionate care. As research advances, the future holds promise of not only improved outcomes but the potential to rewrite the genetic instructions behind this rare but profound immune defect—restoring balance to a system meant to protect, but briefly broken. Close a chapter defined by complexity, but anchor it firmly in hope.

Related Post

Comprehensive Guide to Clubitis: Unlocking Symptoms, Early Detection, and Crucial Insights
Shocking Truths About Your Wrist Lump: Understanding The Phenomenon Of A Cyst That Popped Causes And Care Gnglion Symptoms Nd Cuses Myo Clinic Gnglion Removl

How Much Does a CVS Flu Shot Cost? The Shocking Truth — and Why Now’s the Time to Know Before It’s Too Late

