WhatIsPolymerization? Unlocking the Chemistry Behind Modern Materials
WhatIsPolymerization? Unlocking the Chemistry Behind Modern Materials
From the flexible soles of athletic shoes to the life-saving capabilities of biomedical implants, polymers shape the foundation of countless everyday technologies—yet few truly understand how these materials come to life. At the heart of this transformation lies polymerization, a fundamental chemical process that converts small, reactive molecules called monomers into long-chain polymers with extraordinary properties. Understanding what is polymerization is essential not only for chemists and engineers but also for anyone interested in the materials driving innovation across industries.
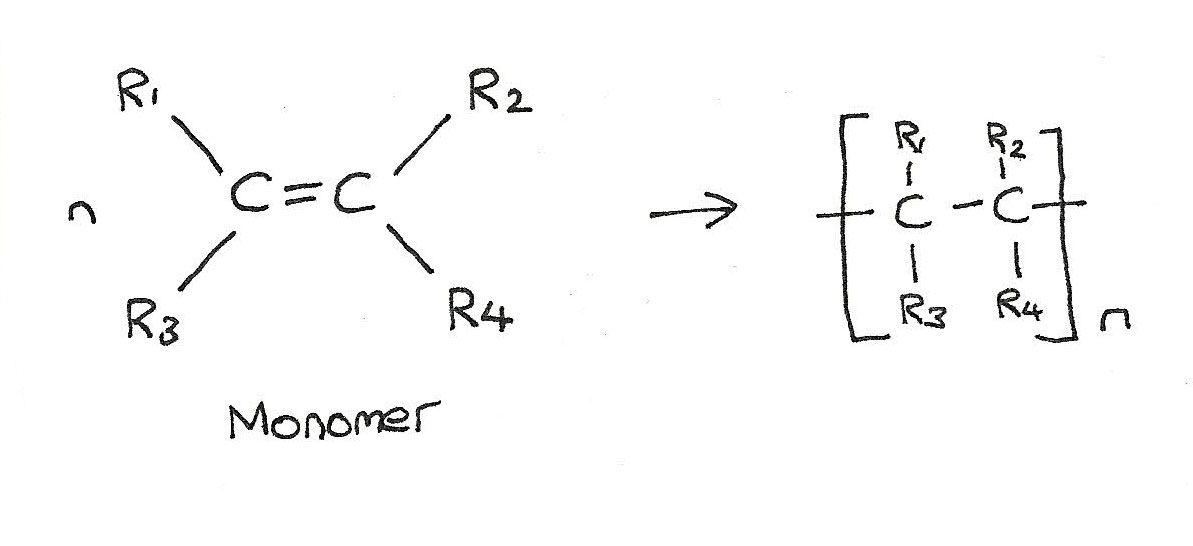
Polymerization is the reaction mechanism by which monomers—typically simple organic compounds with double bonds or reactive end groups—combine to form larger, repetitive macromolecules. The result is a substance with unique mechanical strength, thermal stability, and chemical resistance, depending on the monomers and polymer architecture. This process, while conceptually straightforward, encompasses a wide range of mechanisms and is the cornerstone of manufacturing processes in the plastics, textiles, coatings, and pharmaceutical sectors.
There are two primary pathways of polymerization: addition (or chain-growth) polymerization and condensation (or step-growth) polymerization. In addition polymerization, monomers with unsaturated bonds—such as ethylene or styrene—link together without releasing byproducts, growing rapidly into long chains as catalysts or initiators trigger chain propagation. This method enables fast, efficient production of polymers like polyethylene and polystyrene—materials ubiquitous in packaging and consumer goods.
Condensation polymerization, in contrast, involves the stepwise bonding of monomers with the elimination of small molecules such as water or methanol.
Polyesters, polyamides (nylon), and polyurethanes form through this process, where reaction byproducts remove parts of monomers, gradually building complex structures. The trade-off is longer reaction times and higher sensitivity to process conditions, but the outcome delivers tailored functionalities for demanding applications.
Beyond these two fundamental modes, polymerization diverse into specialized techniques that allow precise control over polymer structure and performance. Living polymerization techniques, for instance, enable scientists to "live" the reaction—meaning chain growth continues until monomer depletion—yielding polymers with narrow molecular weight distributions and predictable architectures.
This precision enhances material properties such as toughness, transparency, and thermal stability. Meanwhile, controlled radical polymerization methods, including atom transfer radical polymerization (ATRP) and reversible addition-fragmentation chain transfer (RAFT), further refine the ability to design polymers with customized architectures—branching, stars, and block structures—unlocking advanced behaviors for cutting-edge applications.
What makes polymerization truly remarkable is its adaptability across scales—from laboratory synthesis to industrial-scale production. Each stage demands meticulous optimization of temperature, pressure, catalysts, and monomer purity to ensure consistent quality and performance.
For example, the production of high-density polyethylene (HDPE) for milk jugs and pipeline internals relies on carefully managed addition polymerization under controlled conditions to achieve either a dense, rigid structure or a more flexible form. Similarly, nylon-6,6 used in clothing and automotive components emerges from a condensation reaction between hexamethylenediamine and adipic acid, demonstrating how chemical design directly translates into functional outcomes.
PHPArray<"polymerization applications"="packaging, medical devices, textiles, advanced coatings, electronics"=">
In the medical field, polymerization enables life-saving innovations. Biodegradable polymers like polylactic acid (PLA), synthesized via ring-opening polymerization of lactide monomers, form dissolvable sutures and drug-delivery systems.
Their controlled degradation rate ensures timely breakdown in the body, eliminating the need for surgical removal. In electronics, conductive polymers produced through oxidative polymerization—such as polyaniline and polypyrrole—pave the way for flexible displays, sensors, and wearable devices, blending materials science with digital advancement.
Environmental considerations now shape polymerization research, driving the development of sustainable alternatives. Innovations include bio-based monomers derived from renewable feedstocks like corn starch or sugarcane, and green polymerization methods that minimize hazardous solvents and energy use.
Catalytic systems emphasizing atom economy and recyclability reflect the industry’s commitment to reducing plastic pollution and carbon footprints.
Advances also extend to nanotechnology, where microemulsion and living polymerization techniques fabricate nanoparticles and nanostructured polymers precisely engineered for targeted drug delivery, imaging, or smart material applications.What is polymerization, then? It is the silent alchemy transforming raw molecules into versatile, dynamic materials that define the functionality of modern life. From nanoscale drug carriers to large-scale infrastructure, the process underpins progress across disciplines.
As science advances, polymerization continues evolving—faster, smarter, greener—ushering in a new era of materials with unprecedented capabilities.
Understanding what is polymerization reveals it not merely as a chemical reaction but as the foundation of technological innovation. With each link in the molecular chain, humanity gains new tools to shape the future—one polymer at a time.


Related Post

Mastering Unit 1 Test Geometry Basics Part 2: Short Answers That Define Geometric Fluency

