WhatIsAConstitutionalIsomer? Unlocking the Secrets of Structure-Driven Molecular Identity
WhatIsAConstitutionalIsomer? Unlocking the Secrets of Structure-Driven Molecular Identity
In the intricate world of organic chemistry, seemingly identical compounds can exhibit profoundly different behaviors—driven not by differing elemental makeup, but by the precise arrangement of atoms in space. This phenomenon lies at the heart of constitutional isomerism, where molecules share the same molecular formula yet diverge in structural connectivity, generating distinct chemical and physical properties. Understanding what a constitutional isomer is is essential for chemists, pharmacologists, and material scientists navigating the molecular basis of reactivity and function.
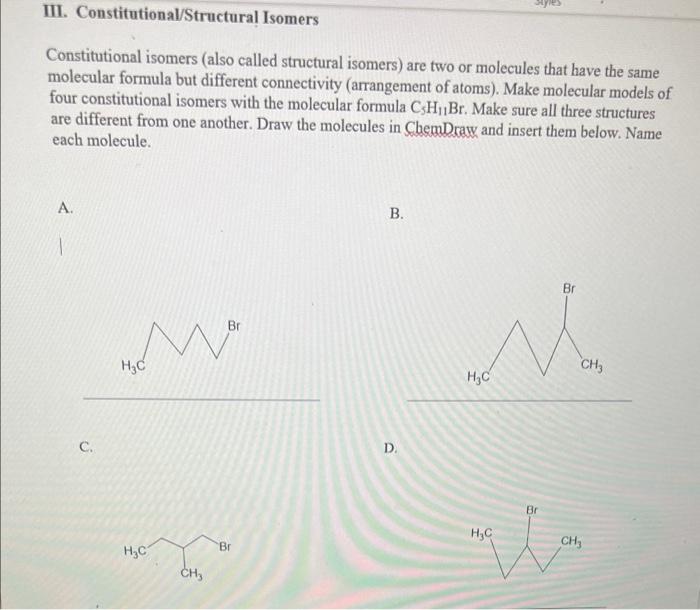
A constitutional isomer—also known as structural isomer—is any pair of compounds that possess the same molecular formula but differ in the sequence or bonding pattern of their atoms. Unlike stereoisomers, which share identical connectivity but vary in spatial orientation, constitutional isomers reflect fundamental distinctions in how carbon atoms and other atoms link together. For example, ethanol (C₂H₆O) and dimethyl ether (CH₃OCH₃) are constitutional isomers: both contain two carbons, two hydrogens, and one oxygen, yet bond differently—forming an alcohol versus an ether linkage.
This variation in atomic arrangement drastically influences physical and chemical properties.
Take butane (C₄H₁₀) and isobutane—both four carbons, ten hydrogens—but butane is a straight-chain alkane, while isobutane features a branched structure. The difference affects boiling points, reactivity in combustion, and application in industrial chemistry. This principle extends across molecular scales—from simple alkanes to complex biological molecules like carbohydrates and amino acids.
Why Molecular Skeleton Structure Matters: The Core Definition
The defining feature of a constitutional isomer is its distinct carbon skeleton or central framework.A carbon skeleton refers to the primary chain of carbon atoms connecting the molecule’s corners and branches. Changing the connectivity—such as creating a double bond where another forms, or shifting branching points—alters the molecule’s topology. According to the American Chemical Society (ACS), “constitutional isomers differ in the order in which atoms are bonded, fundamentally reshaping molecular identity without altering elemental composition.” This structural reconfiguration can transform a stable hydrocarbon into a reactive epoxide, or convert a polar alcohol into a nonpolar ether.
Isomerism through connectivity is not mere curiosity—it governs stability, reactivity, and function at the molecular level. For instance, glucose and fructose, both C₆H₁₂O₆, differ in ring formation: glucose forms a six-membered pyranose ring, while fructose links via a five-membered furanose structure. This difference dictates how each sugar interacts with enzymes, influencing roles in metabolism and energy storage.
Such distinctions underscore why identifying constitutional isomers is critical in drug design, polymer chemistry, and biochemical research.
Real-World Impact: From Pharmaceuticals to Materials Science
The practical significance of constitutional isomers manifests across multiple scientific domains. In medicinal chemistry, subtle structural rearrangements can turn a life-saving drug into an inactive compound or introduce toxic side effects. The infamous example of thalidomide—where one isomer acted as a sedative but another caused severe birth defects—epitomizes this stakes.Modern pharmaceutical screening rigorously evaluates isomer forms to ensure safety and efficacy.
In materials science, constitutional isomers influence macroscopic properties. Polyethylene and polypropylene, though both hydrocarbons (C₂H₄)ₙ, differ in branching and thus strength, flexibility, and melting points—properties engineered for applications from packaging to automotive parts.
Similarly, dendrimers—highly branched macromolecules—rely on precise isomeric control to achieve targeted drug delivery or nanoscale architecture.
Even in planetary chemistry, isomerism shapes atmospheric and surface processes. Hydrocarbons found on Saturn’s moon Titan exhibit complex isomeric distributions influenced by cosmic radiation, informing models of prebiotic chemistry.
These examples highlight how structural isomerism bridges atomic-level intricacies with planetary-scale phenomena.
Identifying Isomers: Techniques and Challenges
Detecting constitutional isomers demands sophisticated analytical and computational tools. Nuclear Magnetic Resonance (NMR) spectroscopy stands as a cornerstone, revealing distinct chemical shifts and coupling patterns that signal different carbon arrangements. Infrared (IR) spectroscopy complements this by identifying functional group vibrations unique to each isomer.Mass spectrometry further aids by mapping molecular ion peaks and fragmentation patterns, enabling precise structural inference.
Yet, identifying constitutional isomers—especially among large or dynamic molecules—remains challenging. Isomers with similar energies may interconvert rapidly, complicating analysis.
Computational chemists now leverage advanced algorithms, such as molecular dynamics simulations and quantum-chemical calculations, to predict stable isomeric forms and validate experimental findings. These tools are indispensable in high-throughput screening and rational drug design.
Case Studies: When Structure Converges, Purpose Diverges
Consider the oxyacids isomerism: formaldehyde (CH₂O) versus paraformaldehyde (a polymer of repeating formaldehyde units). Though formaldehyde’s molecular formula matches paraformaldehyde’s, the latter’s extended backbone enables cross-linking, forming resins used in adhesives and coatings.This shift from monomer to polymer represents not just structural change, but a transition from reactive gas to durable material.
Another instructive example is trimethylamine versus trimethylamine oxide. The former—a basic, skunk-like gas—is vital in biological signaling, while its oxide form, stable in aqueous solutions, plays roles in antioxidant defense.
These contrasting behaviors stem
![[Solved]: 1.32) Draw Structures for all Constitutional isom](https://media.cheggcdn.com/study/cfe/cfe767dd-c01b-4026-8345-22fc2c011840/image)
![[Solved]: Constitutional Isomers These are constitutional i](https://media.cheggcdn.com/media/877/877aa934-c799-4ee9-aa45-4e2dec3ecd5b/phpd14uyl)

Related Post

Today Is Post Office Open: How America’s Postal Network Powers Daily Life
Jennifer Garner’s Kids: A Legacy of Balance, Resilience, and Authentic Parenting
Bryon Noem: A Comprehensive Guide To His Life, Achievements, And Influence As First Gentleman Launches Initiative On South Dakota's Small Towns

Understanding Jasi Bae: A Comprehensive Guide to the Rising Star in Entertainment

