What Is Dehydration Synthesis?
What Is Dehydration Synthesis?
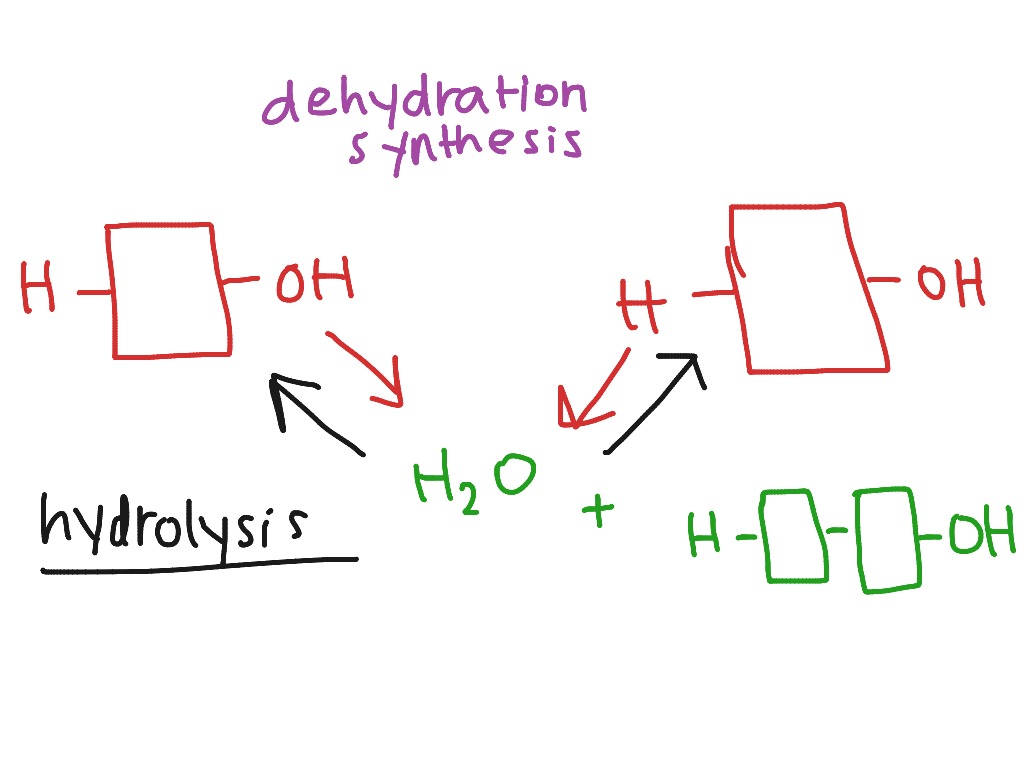
Dehydration synthesis stands at the core of biochemical transformation, enabling the formation of complex molecules through the precise removal of water molecules. This fundamental chemical reaction—where smaller units bond while discarding water—is essential to life’s molecular architecture, constructing proteins, carbohydrates, and nucleic acids from simpler precursors. By tying together atoms with the loss of H₂O, dehydration synthesis fuels the very foundation of biological complexity, linking chemistry to biology in a seamless dance of molecular creation.
At its essence, dehydration synthesis is the biochemical mechanism by which monomers—such as amino acids, monosaccharides, or nucleotides—join together to form larger polymers based on condensation reactions.
Each bond formed eliminates a water molecule, a process succinctly summarized by the principle: *chemical links are forged, water is released*. This reaction contrasts with hydrolysis, its inverse counterpart, in which polymers break down into monomers using water. Understanding dehydration synthesis reveals not just a reaction, but the dynamic engine behind cellular machinery and genetic coding.
How Dehydration Synthesis Works: The Mechanism Explained
Dehydration synthesis relies on functional group reactivity—specifically, the nucleophilic attack on carbonyl carbons by electron-rich atoms.
In the case of amino acids combining to form proteins, the carboxyl group (–COOH) of one molecule reacts with the amino group (–NH₂) of another. As the –OH of carboxyl and the –H of amine join, a water molecule (H₂O) is ejected, and a peptidergic bond—commonly called a peptide bond—forms. This transformation, governed by enzyme catalysis, exemplifies biological precision.
This process unfolds through a stepwise mechanism:
- Activation: The leaving group (often a proton) is removed, enhancing the electrophilicity of the carbonyl carbon.
- Nucleophilic attack: A free lone pair from nitrogen in an amino group displaces the leaving moiety, forming an intermediate.
- Water elimination: The resulting hydroxyl proton departs, stabilizing the new covalent linkage between monomers.
Similar steps occur in glycosylation, where sugar monomers bond via α- or β-glycosidic linkages, shedding water.
Each bond—whether peptide, glycosidic, or phosphodiester—relies on this principle, underscoring dehydration synthesis as the unifying force behind molecular diversity.
Biological Applications: Life’s Molecular Architects
No biological system operates without dehydration synthesis. In protein biosynthesis, amino acids undergo repetitive condensation to assemble chains that fold into functional enzymes, structural scaffolds, and signaling molecules. Without this unceasing bond formation, no metabolic pathway could sustain life.
Enzymes such as aminoacyl-tRNA synthetases orchestrate these reactions, ensuring precision at the atomic scale.
Carbohydrate metabolism illustrates another scale of application. Genetic polymers like DNA and RNA are built through dehydration synthesis, with nucleotide monomers joining via phosphodiester bonds that release water. Each strand of double-stranded DNA, a master blueprint of hereditary information, owes its stability to billions of these condensation events.
Even energy-rich molecules, such as ATP, leverage dehydration synthesis during their formation, storing potential energy in high-energy phosphate bonds.
Polypeptide folding, a critical step in protein functionality, also hinges on the covalent framework established by dehydration synthesis. Once linked, secondary structures (α-helices, β-sheets) emerge, driven partly by the energy released during bond formation. Thus, dehydration synthesis not only constructs molecules but primes them for dynamic biological roles—from catalysis to genetic replication.
Energy Dynamics and Enzymatic Control
Dehydration synthesis is inherently energy-intensive, requiring activation energy to overcome thermodynamic barriers.
The dehydration step converts a labile anhydride bond into a stable but energetically labile linkage—one poised to fuel further reactions or drive downstream processes. This energy investment is offset by enzyme-catalyzed catalysis, which lowers activation energy and accelerates bond formation with remarkable efficiency.
Enzymes play a decisive role, functioning as catalytic hubs that align substrates and stabilize transition states. For instance, ribosomes reduce the entropy penalty of amino acid assembly by precisely positioning monomers, while DNA polymerases ensure directional bond formation with matching bases.
Without such biological facilitators, dehydration synthesis would proceed too slowly or randomly to support complex life systems.
The strategic use of enzymes reflects evolution’s optimization: by harnessing dehydration synthesis within tightly regulated environments, cells achieve both speed and specificity. This balance enables



Related Post

Don Slater Net Worth: The Rising Trajectory of a Tech Entrepreneur’s Financial Legacy

Gladys Knight Net Worth: A Rising Star Built on Soul, Resilience, and Decades of Legendary Success

Comcast Xfinity Email Sign-In Simplified: Seamless Email Login Makes Comcast Access Effortless

