What Experiments Did Niels Bohr Perform That Revolutionized Quantum Physics
What Experiments Did Niels Bohr Perform That Revolutionized Quantum Physics
Niels Bohr’s experimental and theoretical hybrid approach to atomic structure reshaped our understanding of the quantum world. Though best known for his postulate defining electron orbits, Bohr’s contributions were deeply grounded in empirical inquiry, bridging observational data with bold theoretical leaps. His experiments—conducted primarily in the early 20th century—provided critical confirmations of quantum mechanics, establishing principles still foundational to modern physics.
What transpired in Bohr’s laboratories and thought experiments laid the groundwork for how electrons behave, energy is emitted and absorbed, and the nature of atomic states itself.
The Bohr Model: A Departure from Classical Physics
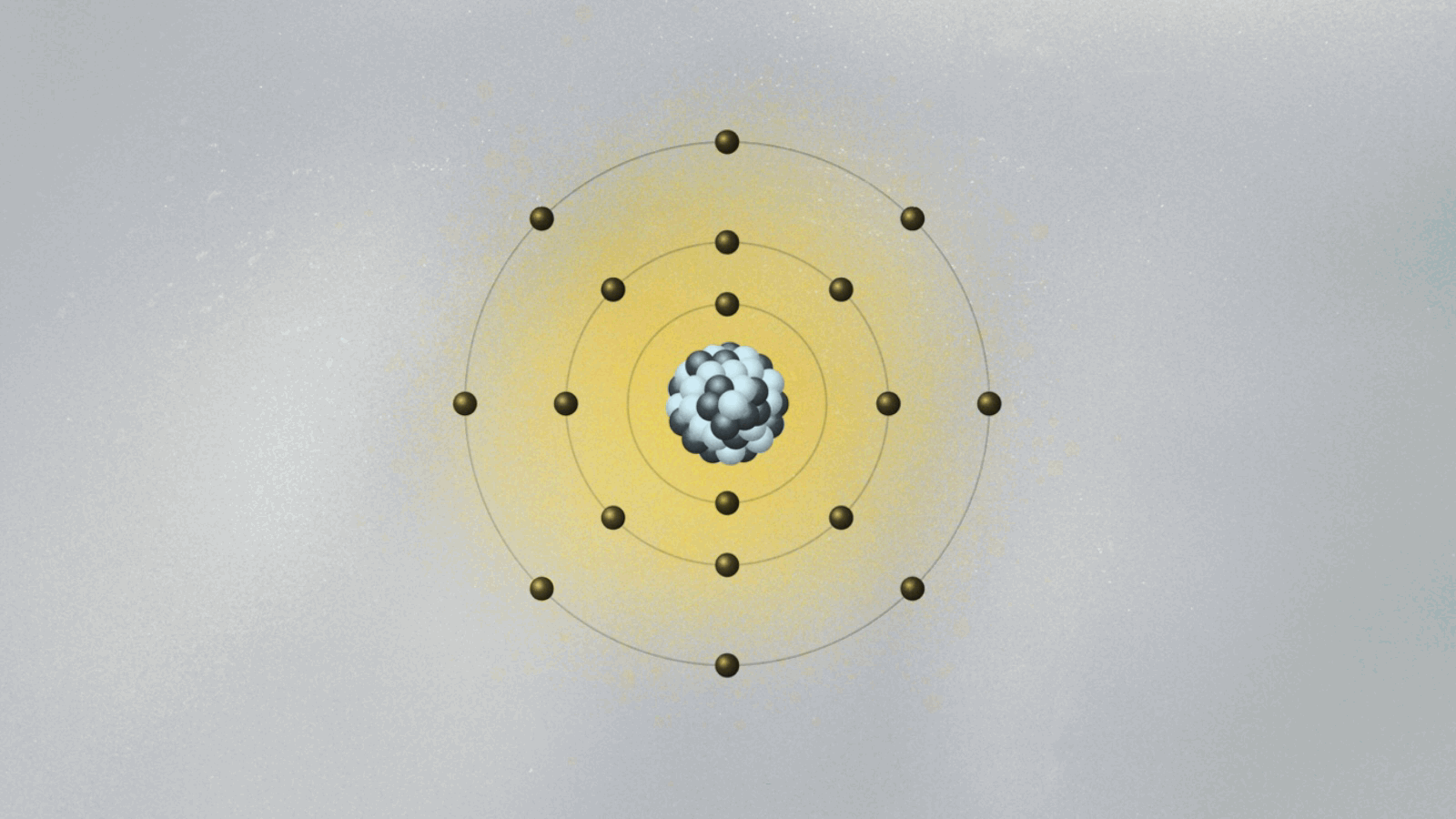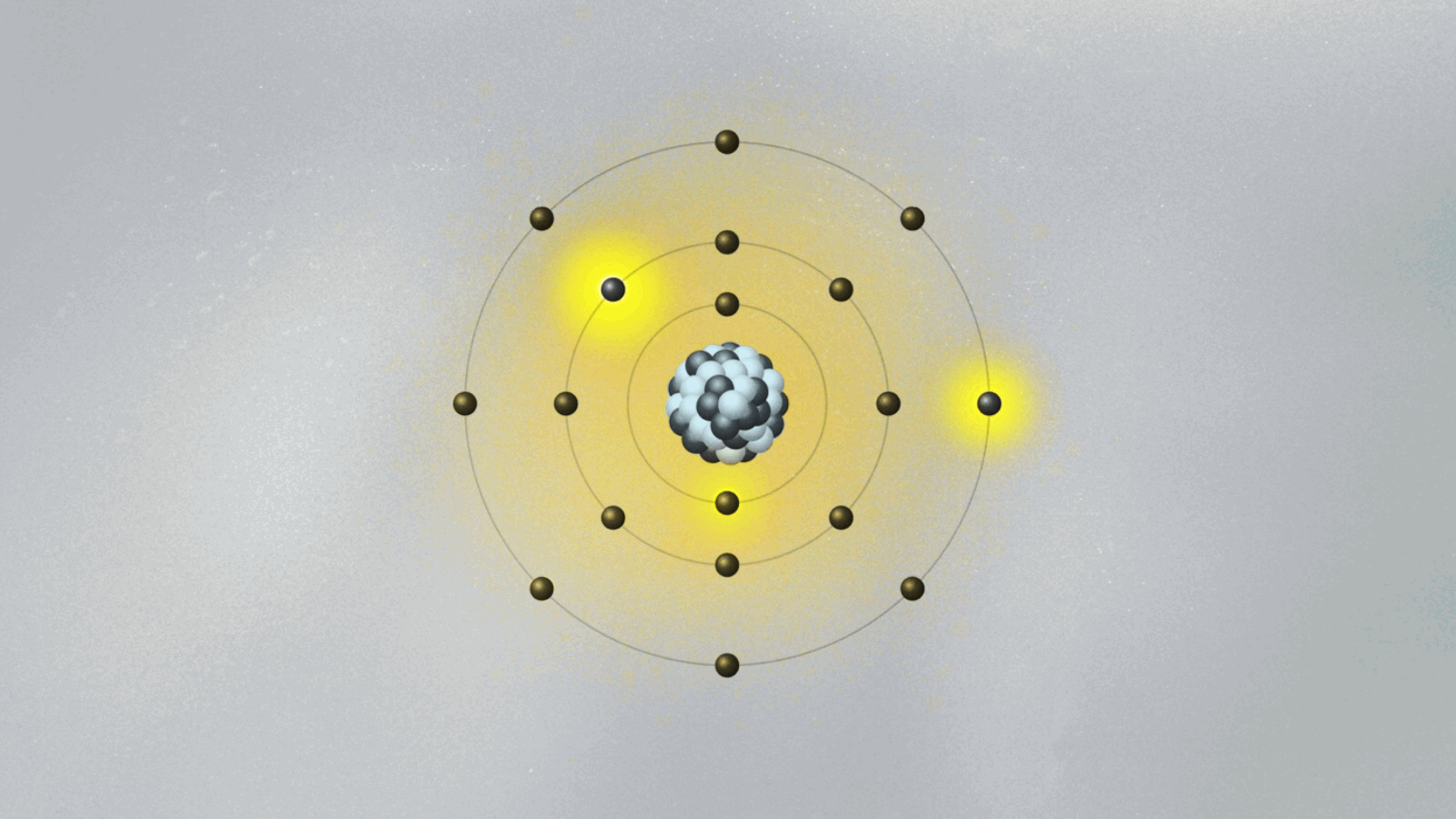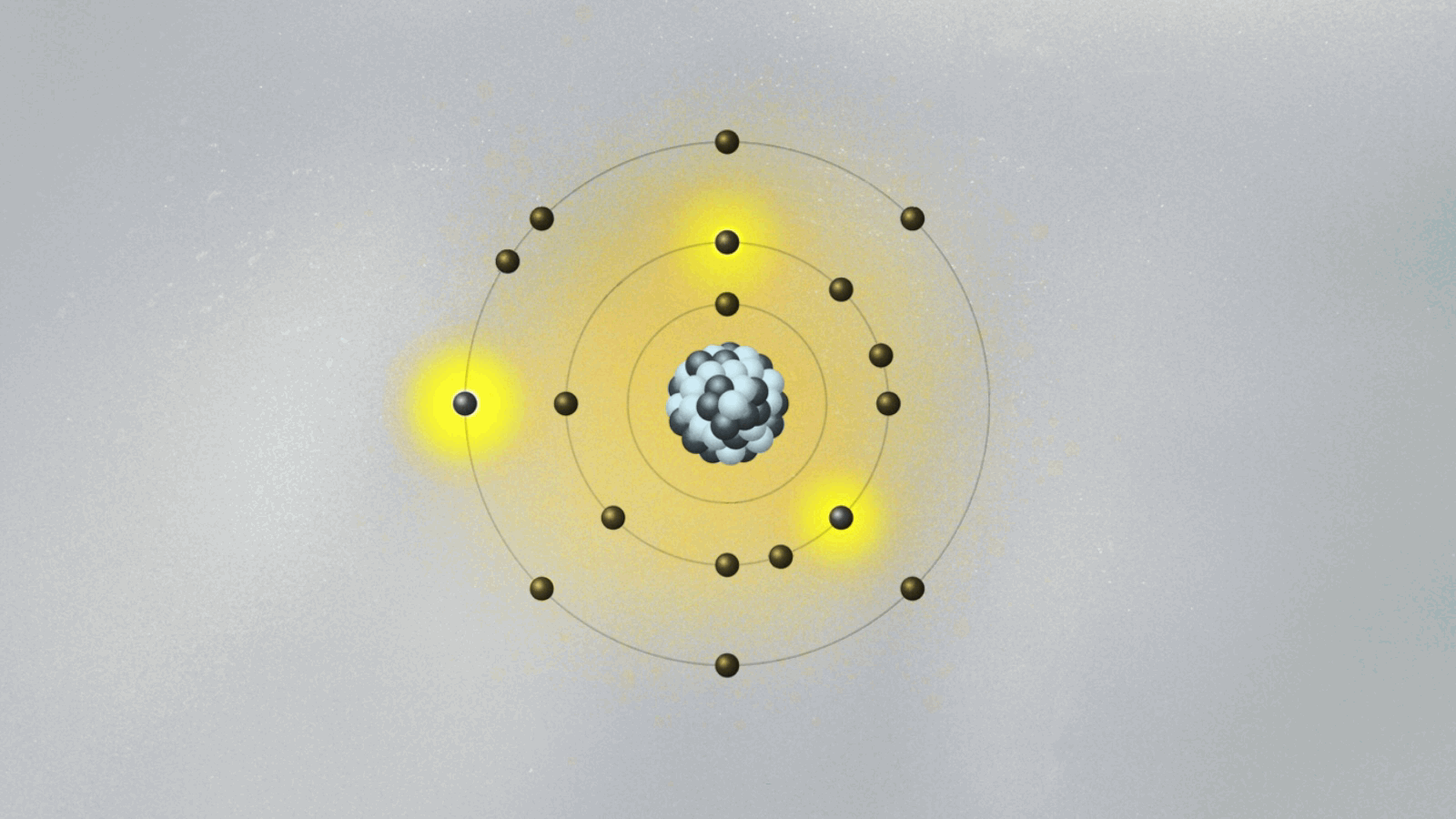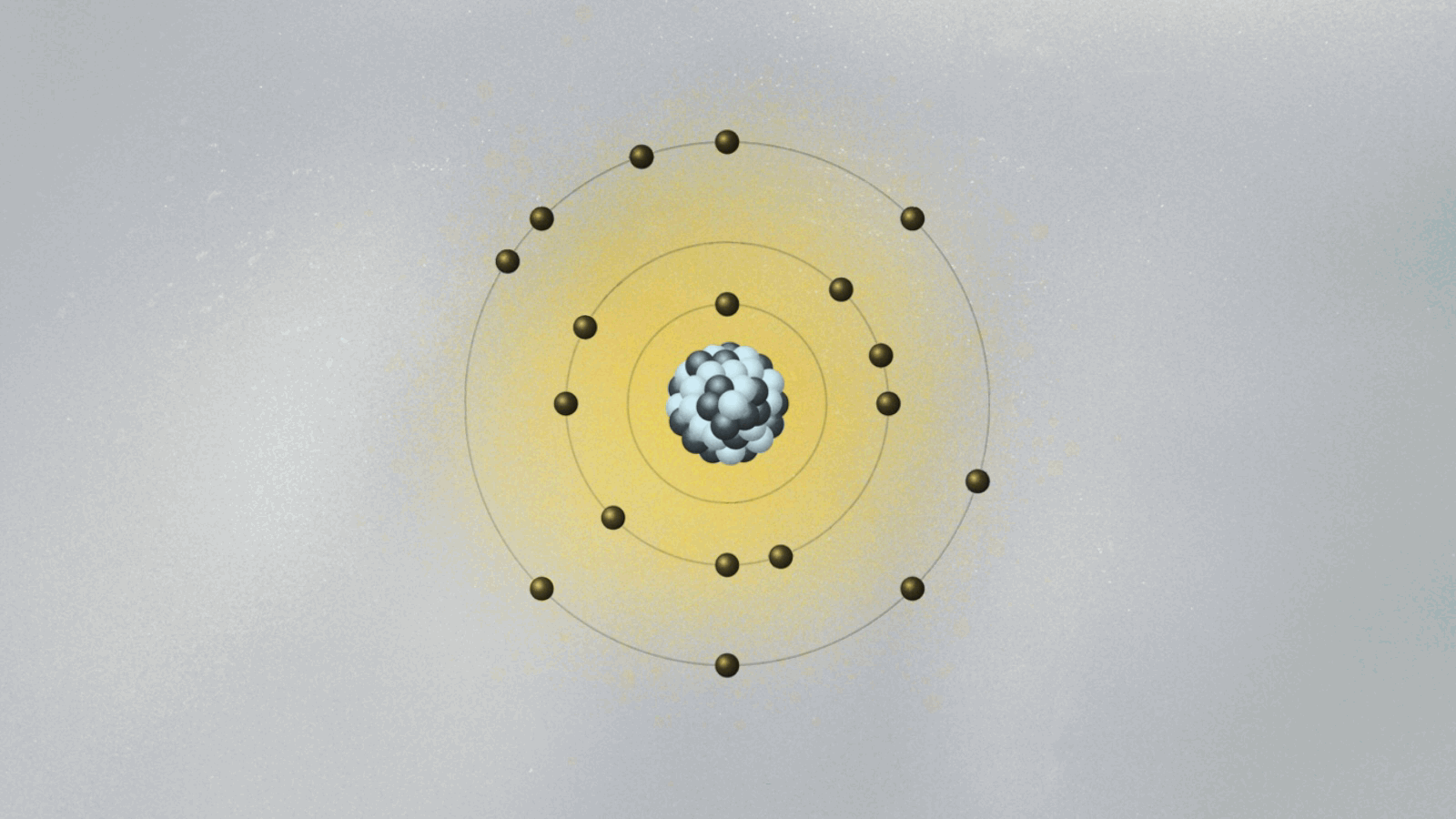
Bohr’s most celebrated model—the Bohr atomic model—emerged not just from theory, but from direct engagement with experimental data, particularly the spectral lines emitted by hydrogen. Prior models failed to explain why atoms emitted light in discrete frequencies, a phenomenon central to spectroscopy.- In 1913, Bohr formalized his model using three key postulates: • Electrons orbit the nucleus in fixed, quantized energy levels without radiating energy. • Electrons emit or absorb photons only when transitioning between orbitals, with energy corresponding to the difference between levels: ΔE = hν, where h is Planck’s constant and ν is the emitted frequency. • Each allowed orbit satisfies angular momentum quantization: L = nħ, where n is an integer and ħ is the reduced Planck constant.
This framework explained the Balmer, Lyman, and Paschen series observed in hydrogen’s emission spectrum—spectral lines previously inexplicable under classical electrodynamics. Bohr’s use of spectroscopic data to validate his quantum-inspired orbits marked a turning point in atomic science.
Bohr’s model wasn’t flawless—quantum electrodynamics later refined it—but it offered a testable, predictive framework that merged observation with innovative synthesis.
The experiments were conceptual as much as physical, demanding indirect verification through spectral analysis rather than direct imaging of electrons, which remained beyond technological reach at the time.
The Complementarity Principle: Beyond Measurement and Reality
Beyond atomic transitions, Bohr pioneered deeper philosophical experiments in quantum theory through the introduction of the complementarity principle. This conceptual framework arose from experimental ambiguities inherent in phenomena like the double-slit experiment, where particles exhibit both wave-like interference and particle-like localization—never simultaneously in the same experimental setup. Bohr argued that such contradictory behaviors were complementary aspects of quantum reality, not mutually exclusive truths.The act of measurement itself determines the observable outcome: - Observing which path a particle takes destroys interference patterns, while measuring wave properties obscures concrete trajectory. - “The wholeness of the experimental outcome is greater than the sum of its parts,” Bohr asserted, highlighting the irreducible role of the observer and apparatus in defining physical reality. This principle transformed quantum interpretation, emphasizing that experimental design inherently shapes what can be known—and that quantum systems resist classical dichotomies.
Bohr’s invitation to reconcile seemingly opposing quantum features challenged physicists to embrace epistemic humility, turning experimental limitations into profound insights about nature’s underlying structure.
The Bohr-Einstein Debates: Experimental Logic vs. Determinism
Bohr’s experimental reasoning placed him at the heart of the famous Bohr-Einstein debates, where theoretical rigor collided with philosophical clarity. Einstein questioned whether quantum mechanics provided a complete description of physical reality, favoring deterministic hidden variables.Bohr countered with empirically grounded thresholds, emphasizing that quantum phenomena could only be addressed through carefully designed experiments with quantifiable outcomes. In discussions over entanglement and measurement outcomes—later formalized in the EPR paradox—Bohr insisted verification required alignment with statistical regularities observed in experiments, not intuitive realism. He maintained that quantum mechanics’ predictive power stemmed not from metaphysical assumptions but from its consistency within experimentally confirmed frameworks.
His defense of quantum indeterminacy was never abstract: it emerged from interpreting spectral statistics, measurement precision limits, and interference phenomena as critical benchmarks for experimental validation.
The Copenhagen Interpretation and Experimental Verification
Bohr’s leadership in advancing the Copenhagen interpretation cemented his belief that quantum experiments must define physical truth. Experimentation became the arbiter of theory, with concepts like wavefunction collapse interpreted through observable measurement results. His lab in Copenhagen became a crucible where experimental anomalies—such as anomalous energy shifts in fine-structure spectra—tested theoretical predictions.Bohr insisted that any viable atomic model must reconcile with these empirical outcomes, shaping how physicists approached spectroscopy, quantum transitions, and atomic stability.
This experimental-first philosophy ensured quantum mechanics evolved not just from elegant mathematics, but from what could, and could not, be observed.
Legacy: Experiments as the Compass of Quantum Discovery
The experiments Niels Bohr conducted—whether theoretical thought experiments verified through spectroscopic data, or conceptual tests of measurement limits—reshaped physics by anchoring quantum theory in empirical reality. His fractional orbits explained spectral lines precisely, his complementarity principle redefined what observables could mean, and his debates sharpened the philosophy of experiment in quantum domains.Bohr’s insight—that quantum mechanics is not merely a mathematical construct, but a system validated through carefully designed experimental inquiry—remains a cornerstone of modern atomic and particle physics. Today’s tools, from laser spectroscopy to quantum computing experiments, trace their lineage to Bohr’s insistence that nature reveals herself only through rigorous, intelligent experimentation.
In the quiet diligence of isotope separation or spectral analysis, Bohr transformed human understanding—one experiment, one fusion of thought and measurement, at a time.


Related Post

Elevate Growth: Aceleration City Is Redefining Urban Innovation
Scrutinizing the Upheaval of 9X Movies
Kate Merrill WBZ Bio Wiki Age Height Husband Sister Salary and Net Worth

