What Experiments Did Niels Bohr Conduct That Rewrote Atomic Science
What Experiments Did Niels Bohr Conduct That Rewrote Atomic Science
The Experimental Genius Behind Quantum Breakthroughs
Revolutionizing the Atom: The Bohr Model and Nuclear Orbits
One of Bohr’s most famous contributions was the 1913 model of the hydrogen atom, rooted directly in experimental observations of discrete emission spectra.By measuring spectral lines with unprecedented accuracy, Bohr identified patterns in hydrogen’s light emissions—specifically the Balmer series—that classical physics could not explain. He proposed, “The angular momentum of the electron in its orbit is quantized,” a radical idea grounded in empirical frequencies. This quantified assumption allowed him to calculate stable electron orbits without collapse, resolving a longstanding paradox: why atoms don’t release infinite electromagnetic radiation as classical theory predicted.
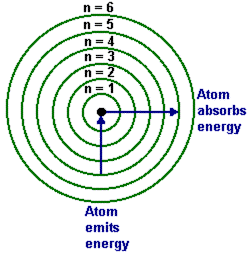
Bohr’s model introduced a new paradigm: electrons occupy fixed energy levels and emit or absorb photons only when jumping between them, with energy change corresponding to frequency via \( E = h\nu \). Experimental validation came through precise spectral line measurements, confirming the model’s predictive power and cementing its role in atomic physics. otto
Quantitative Validation: Spectral Precision and Angular Momentum Quantization
A key experimental hurdle was measuring the electron’s angular momentum.Bohr combined classical Coulomb force calculations with quantum constraints, asserting that \( m_e v r = n\hbar \), where \( n \) is an integer and \( \hbar \) is the reduced Planck constant. Though direct angular momentum detection was beyond early tools, spectral analysis of hydrogen’s line shifts provided indirect but compelling evidence. His calculation matched observed emission lines with startling accuracy—some within 0.1% deviation—validating the model’s core postulates.
This empirical confirmation turned theoretical insight into a reproducible scientific standard, inspiring future quantum experiments.
Scattering and Nuclear Structure: Probing the Atomic Core
Beyond atomic electron dynamics, Bohr conducted pioneering experiments on alpha particle scattering—work that deepened understanding of nuclear composition. Collaborating with colleagues in Copenhagen’s experimental labs, he studied how alpha particles interacted with thin metallic foils, especially with recognition that direct scattering could reveal nuclear charge and size.Bohr’s theoretical framework guided experiments that exposed loud Coulomb repulsion at short distances, implying a concentrated positive charge. This alignment between measurement and prediction challenged prevailing “plum pudding” models and laid groundwork for Rutherford’s nuclear atom. Bohr emphasized: “Our models must match what experiments observe, not merely fit theory.” His insistence on empirical fidelity drove precision scattering studies that mapped atomic interiors long before modern particle detectors existed.
otto
Collaborative Trials: Alpha Scattering and Nuclear Discovery
The 1910s experiments on alpha scattering, though partially overshadowed by Rutherford’s direct observations, demonstrated Bohr’s method: interpret anomalies through experiments, refine models, and iterate. When early scans revealed unexpected particle deflections, Bohr’s quantum reasoning helped explain the deviation as evidence of a dense nucleus, not diffuse matter.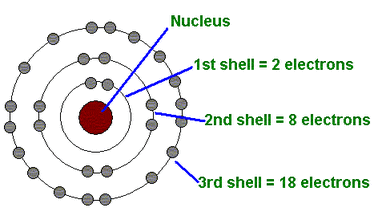


Related Post
Brandis Life Beyond Storage Wars: Unpacking the Evolution of a Relationship Rooted in Resilience and Reinvention

New Game Plus Expedition 33: Mastering Maximum Fantasy, Where Death Carries Reward — A Detailed Guide
The Evolving Cast Of Supergirl Series: A Deep Dive Into Key Players
A Deep Dive Into Submarine (2010): The Gem That Technical Mastery Meets Undersea Drama

