What Charge Is A Proton: The Electrically Positive Core of Atomic Structure
What Charge Is A Proton: The Electrically Positive Core of Atomic Structure
The proton, a fundamental subatomic particle, carries a positive electric charge that lies at the heart of chemistry, physics, and the very building blocks of matter. With a charge of exactly +1.602 × 10⁻¹⁹ coulombs, this positively charged particle defines the identity of atomic nuclei and governs the behavior of elements across the periodic table. Understanding the proton’s charge is not merely an academic detail—it is essential to decoding the atomic forces that shape chemistry, materials science, nuclear physics, and even life itself.
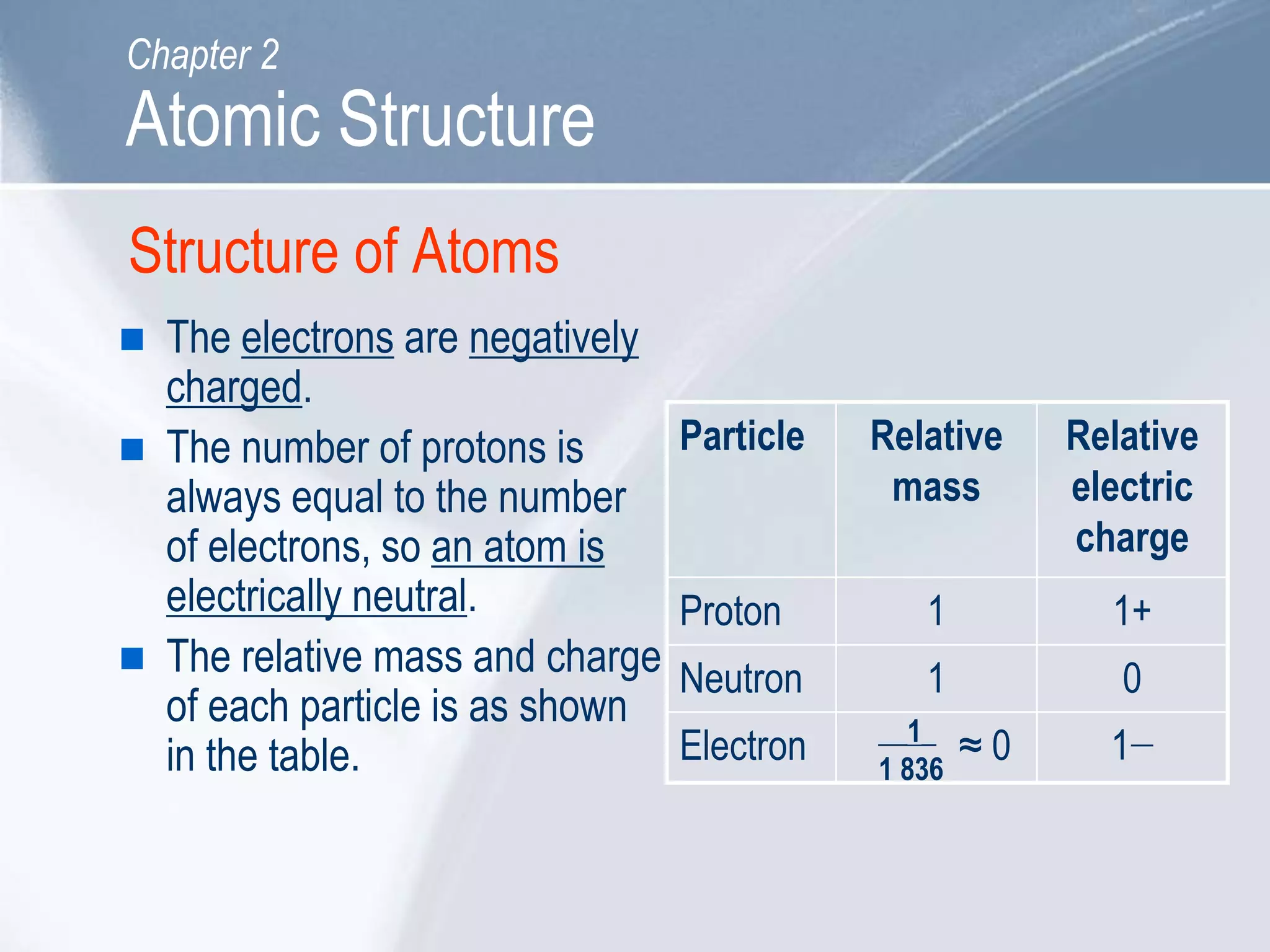
Each proton resides at the dense center of an atom’s nucleus, where it co-stays with neutrons to form atomic mass. Its positive charge attracts negatively charged electrons, establishing electrostatic balance that defines atomic stability. This balance is the silent foundation of chemical bonding, influencing molecular geometry, reactivity, and material properties.
The precision and universality of the proton’s charge make it one of the most reliable constants in natural science.
The Standard Value: 1.602 × 10⁻¹⁹ Coulombs
Determined through meticulous experimental measurements and refined via quantum electrodynamics, the charge of a single proton is defined as precisely +1.602 × 10⁻¹⁹ coulombs. This exact value, established by decades of research, reflects the fundamental structure of electric charge in nature.While modern physics treats charge as quantized—meaning it appears in discrete multiples of this elementary charge—the proton stands as the archetype. This numerical standard plays a critical role in equations governing electromagnetism, particularly Coulomb’s law, which calculates the force between charged particles. The consistency of this value across experiments reinforces the reliability of atomic models and underpins technologies from electron microscopy to semiconductor design.
Historical Discovery and Standardization
The journey to identify the proton’s charge began in the late 19th century with experiments involving cathode rays and early electrically conductive materials. However, the definitive breakthrough came in 1917 when Ernest Rutherford used alpha particle scattering to infer the proton’s existence and intrinsic properties. Yet, precise charge measurement demanded next-generation techniques.
In the 1920s and 1930s, Millikan’s refinements of the oil-drop experiment, combined with advanced spectroscopic analysis, enabled scientists to confirm the magnitude of the proton’s charge with unprecedented accuracy. The value was formally adopted in international standards, becoming a defining constant in physics and chemistry curricula worldwide.
The Electrostatic Significance of the Proton’s Charge
A proton’s +1.602 × 10⁻¹⁹ C charge exerts a direct, measurable influence on its environment. In atoms, the electrostatic attraction between protons and orbiting electrons stabilizes atomic structure. Without this balance, nuclei would disintegrate under internal repulsive forces, and complex matter could not exist.This charge also explains phenomena across scales. In chemistry, it drives ionic bonding—where electrons transfer between atoms—giving rise to salts, acids, and bases. In materials science, controlled manipulation of protons informs proton exchange membrane fuel cells, crucial in clean energy applications.
In biology, proton gradients power cellular respiration and nerve signal transmission, illustrating the charge’s life-sustaining role. Even in particle physics, the proton’s charge defines its interaction with electromagnetic fields—a trait shared by all quarks (specifically the up quark inside the proton) but isolated in impact due to its net positive value. While every atom’s identity hinges on its nuclear protons—each contributing +1.602 × 10⁻¹⁹ C—their collective behavior forms the foundation of matter as we know it. The consistency and accuracy of the proton’s charge value underscore not only the precision of modern science but also the profound interconnectedness of electricity and matter. Every time we observe chemical reactions, power devices, or explore subatomic particles, we engage with the well-defined nature of this tiny, positively charged particle—an enduring testament to the elegance of natural law.



Related Post
Shotzi Blackheart Brandi Lauren Have Fun With Report About Their Relationship
Kelly Emberg: Unpacking the Career Trajectory of a Sports Media Icon
Unmasking Hailey Sigmond: The Shocking Secret That Redefined Her Public Identity
SoFi Stock Drops: Analyzing the Factors Behind Recent Volatility

