What Are the Monomers of Carbohydrates? Decoding the Building Blocks of Life’s Most Abundant Molecules
What Are the Monomers of Carbohydrates? Decoding the Building Blocks of Life’s Most Abundant Molecules
Carbohydrates, the energy powerhouses of biology, owe their prevalence to a simple yet powerful molecular design — a precise alignment of repeating monomer units. At their core, all carbohydrates are composed of identical or varied arrangements of three-carbon monomeric building blocks, primarily glucose, fructose, and galactose. These monomers link together through glycosidic bonds to form disaccharides, oligosaccharides, and polysaccharides, each serving distinct roles across biological systems.
Understanding what constitutes the monomers of carbohydrates reveals not only their structural elegance but also their indispensable function in physiology, nutrition, and biochemistry.
The Fundamental Monomers: Monosaccharides in Molecular Focus
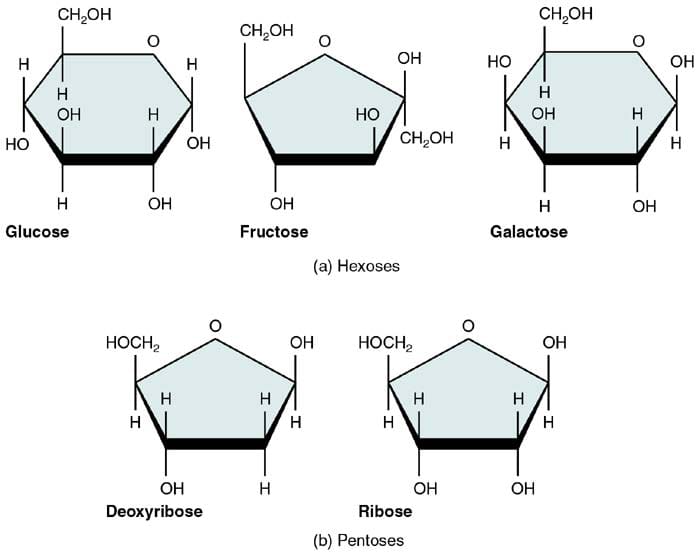
Monosaccharides — literally "simple sugars" — form the singular building blocks of carbohydrates and are defined by their smallest molecular form, incapable of further chemical breakdown without losing carbohydrate identity. These five-carbon sugars dominate biological systems and serve as the essential units for higher carbohydrate structures. The most biologically relevant monosaccharides include: - **Glucose**: The primary energy source for cells, a hexose sugar vital in respiration and metabolic pathways.
- **Fructose**: A ketohexose commonly found in fruits and honey, contributing sweetness and metabolic flexibility. - **Galactose**: Less abundant in free form but critical in milk sugar (lactose), where it pairs with glucose. - **Mannose**: A demethylated glucose variant important in glycosylation processes.
- **Ribose and Deoxyribose**: Five-carbon sugars forming the sugar backbone of RNA and DNA, underscoring their role beyond metabolism into genetic material.
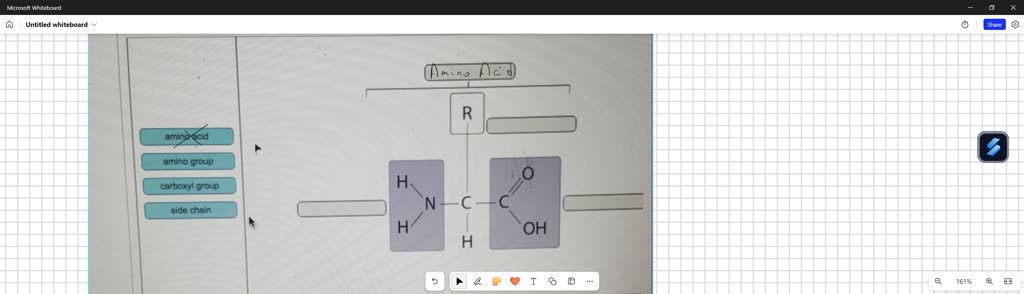
Structural Insight: The Role of Carbon and Hydroxyl Groups Each monomer shares a common structural motif: a central carbon backbone with hydroxyl (-OH) groups at each of the other three outer carbons. The placement and bonding of these groups enable precise chemical reactivity. For instance, glucose’s aldehyde group at carbon-1 facilitates condensation reactions with fructose’s keto group during disaccharide formation — a process central to energy storage in glycogen and starch.
As described by biochemist Dr. Sarah Lin: “The stereochemistry and functional group orientation of these hydroxyls are why monosaccharides are so versatile in forming stable, directional glycosidic linkages.”
Bridging Monomers: From Monosaccharides to Polysaccharides and Disaccharides
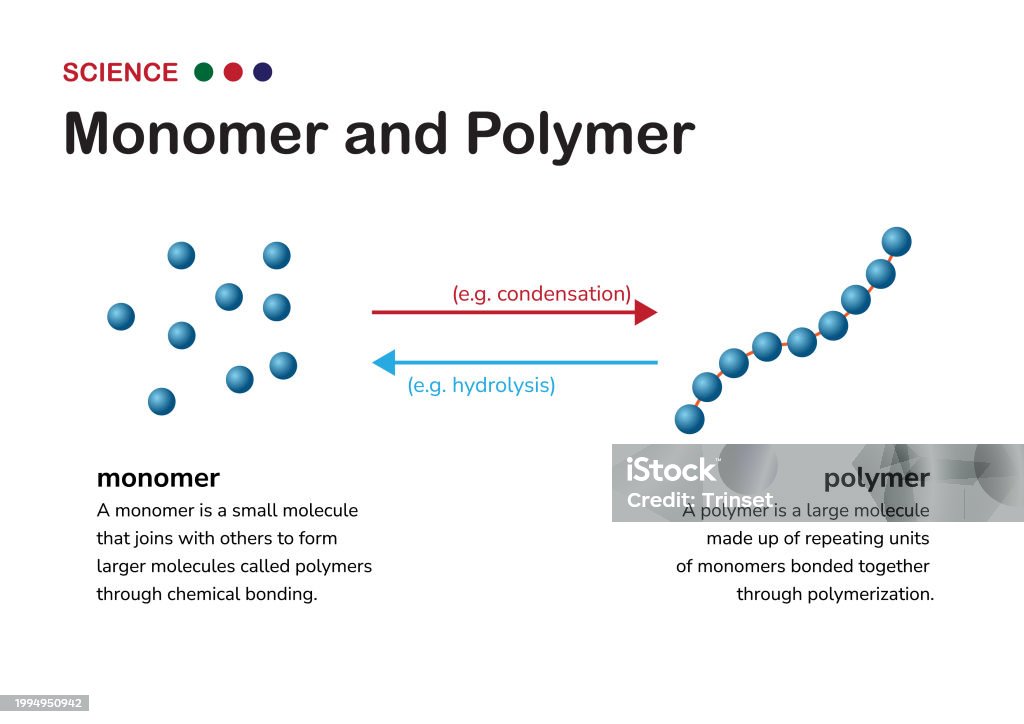
While individual monomers undergo minimal change, their ability to link defines the diversity of carbohydrate functions. When two monomers unite via a glycosidic bond — a linkage formed by releasing a water molecule — they form a disaccharide.
Saccharose (table sugar), for example, arises from glucose and fructose, a combination that enhances stability and transport properties. Lactose andargerose rely on similar linkages, with galactose tethered to glucose in milk and mannose coupled with glucose in lactose. Beyond pairings, chains extend into oligosaccharides (3–10 units) and polysaccharides (100+ units), each engineered for specialized roles.
Polysaccharides such as starch, glycogen, and cellulose illustrate how monomeric repetition translates into macroscopic function.
Starch, the plant’s glucose storage polymer, consists of amylose (linear glucose chains) and amylopectin (branched variants), enabling efficient energy reserves. Glycogen, often called “animal glycogen,” features even greater branching, maximizing rapid glucose release during metabolic demands. Conversely, cellulose — a structural polysaccharide in plant cell walls — arises from linear β-1,4-linked glucose polymers, creating rigid, insoluble fibers that resist enzymatic breakdown in mammals but sustain plant structure.
Nutritional Significance and Metabolic Pathways
Carbohydrate monomers directly influence human nutrition and metabolism.
Glucose serves as the brain’s preferred fuel, transported via blood and metabolized in glycolysis to produce ATP. Excess glucose is stored as glycogen or converted to fat, highlighting its dual role in energy provision and regulation. Fructose, metabolized primarily in the liver, provides an efficient energy source but requires careful intake due to potential impacts on lipid profiles.
The presence of dietary monosaccharides in fruits and dairy supports glycogen synthesis and cellular repair, while indigestible oligosaccharides act as prebiotics, nourishing beneficial gut microbiota.
Furthermore, the linear vs. branched configurations of polysaccharides determine digestibility and glycemic response. Highly branched structures like amylopectin raise blood sugar faster than linear starches, affecting insulin sensitivity and long-term metabolic health.
These nuances underscore how monomer structure cascades into nutritional outcomes, reinforcing the importance of carbohydrate quality in diet.
Industrial and Biotechnological Applications
Beyond biology, carbohydrate monomers power innovation across industries. Glucose-derived ethanol has emerged as a renewable biofuel, while starch-based polymers offer sustainable packaging alternatives. High-purity monosaccharides serve as substrates in fermentation, enabling production of pharmaceuticals, bioactive compounds, and industrial enzymes.
Genetic engineering leverages monomer specificity to develop synthetic glycans for drug delivery or vaccine adjuvants, demonstrating how precise molecular architecture translates into technological leverage.
Advances in carbohydrate chemistry increasingly focus on enzymatic control over monomer assembly, enabling tailored oligosaccharide designs for nutraceuticals and targeted therapies. As the National Institute of carbohydrate research notes: “Mastering monomer monomers unlocks unprecedented control over carbohydrate behavior — from dietary health to material science.”
In summary, the monomers of carbohydrates — glucose, fructose, galactose, and their variants — are not merely chemical units but dynamic architects of life. Their structural consistency, reactivity, and diversity enable the formation of everything from cellular energy stores to structural plant tissues.
Understanding what constitutes the monomers transforms abstract chemistry into tangible insight — revealing how minute molecular arrangements underpin vast biological complexity and human innovation.

Related Post
Komaru: How Danganronpa's 'Normal' Hero Redefined Hope

Rod Stewart Ignites 2025 USA Tour with High-Energy Nights Across Key Cities

