Visceral vs Parietal Pleura: The Hidden Battle Between Life-Sustaining Lining and Structural Barrier
Visceral vs Parietal Pleura: The Hidden Battle Between Life-Sustaining Lining and Structural Barrier
The human chest cavity harbors a deceptively simple yet critically vital structure: a double-layered pleural envelope that separates lung tissue from body wall. At the core of this system lie two distinct types of pleura—visceral and parietal—each with unique anatomical roles, biomechanical properties, and clinical significance. Understanding their differences is essential not only for grasping respiratory physiology but also for diagnosing and managing a spectrum of pulmonary diseases.
While both layers function synergistically to enable lung expansion and stabilize the respiratory cycle, their anatomical origins, sensory innervation, and pathophysiological responses diverge in profound ways.
Anatomical Origins and Forced Separation in the Thoracic Cavity
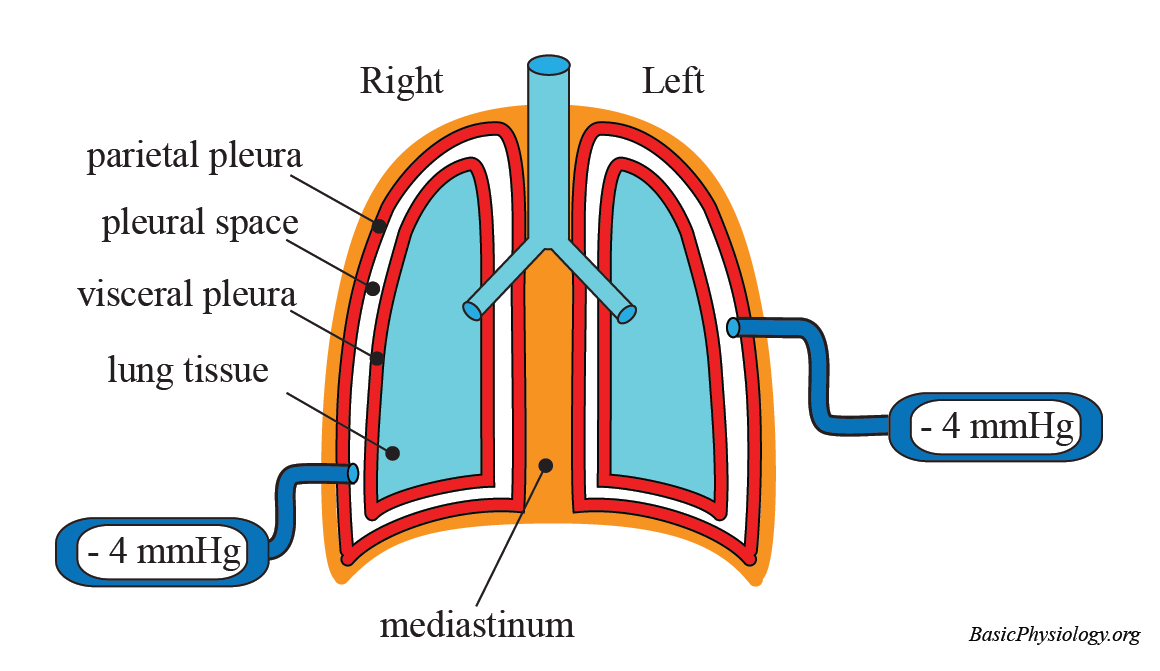
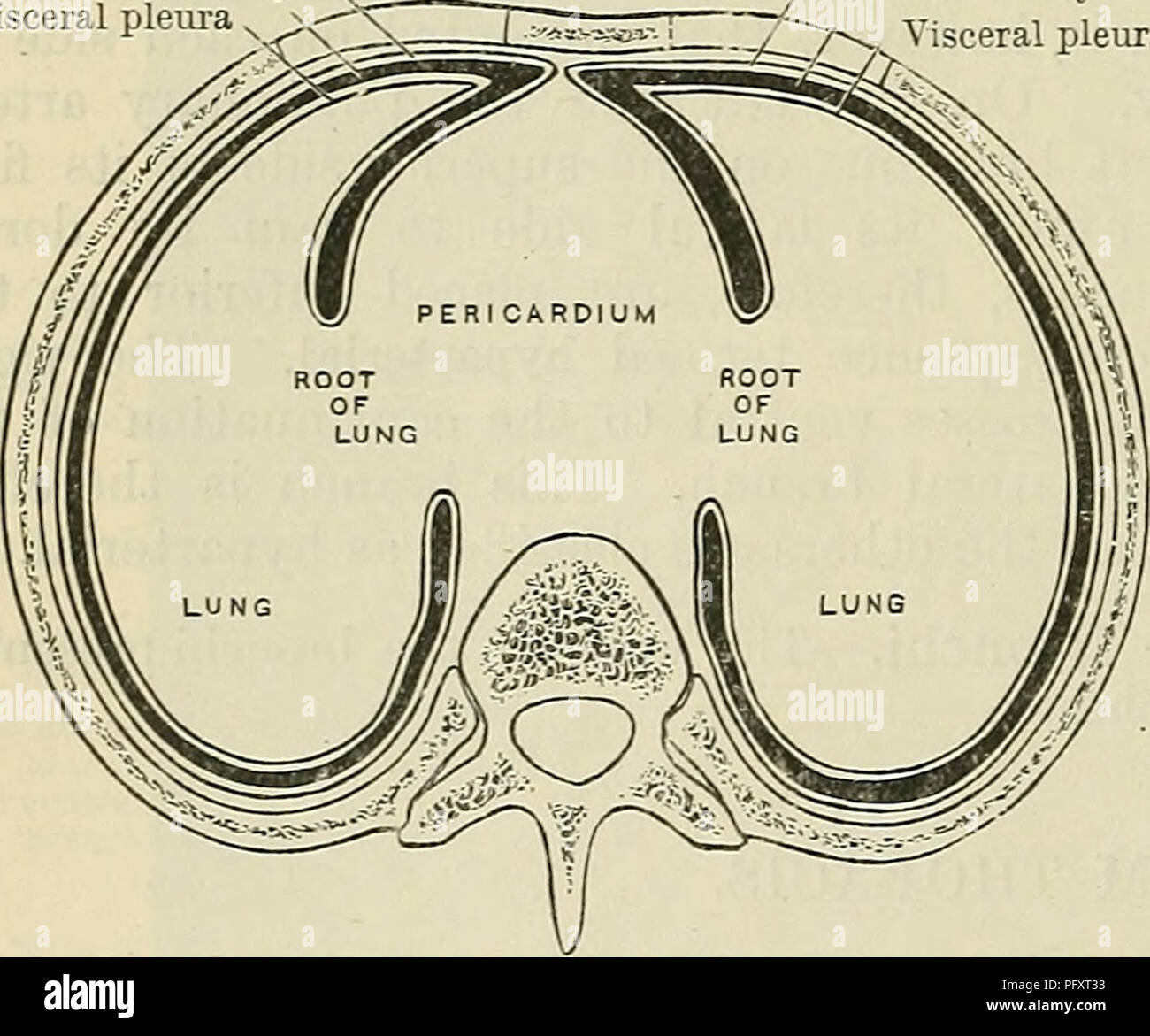
The parietal pleura lines the internal surface of the thoracic wall, diaphragm, and mediastinum, anchoring the lungs to the body’s rigid framework. Journalspan anatomists define it as a serous membrane fused to visceral structures via connective tissue, yet entirely distinct in its development and function.In contrast, the visceral pleura envelops the lungs themselves—adherence to pulmonary parenchyma being its defining feature. “These two layers are functionally unified yet anatomically irreconcilable,” explains pulmonologist Dr. Elena Marquez, “each evolves independently, !=and respond uniquely to injury.” This separation ensures mechanical independence: the lung’s delicate tissue moves freely within the chest, while the parietal layer acts as a stable reference framework, transmitting forces during breathing without compromising lung dynamics.
Differing by origin, their orientations define biomechanical behavior. The parietal pleura follows a layered, laminated configuration along rib surfaces and mediastinal regions, creating a tight, elastic interface. The visceral pleura, concentrated inward on alveoli and bronchial branches, forms a delicate yet resilient barrier that permits constant micro-expansion and contraction.
This structural asymmetry enables the lungs to collapse partially during expiration without tearing, while maintaining a sealed environment critical for efficient gas exchange.
Sensory Innervation and Pain Perception: A Critical Distinction
One of the most clinically consequential differences lies in sensory innervation. The parietal pleura contains robust sensory nerve endings responsive to stretch, inflammation, and irritation—making it the primary source of sharp, localized chest pain when inflamed.This explains why pleuritic pain—steady, stabbing, and exacerbated by breathing—often signals pleural inflammation such as in pneumonia or pulmonary embolism. Conversely, the visceral pleura is sparse in sensory nerves, rendering it largely insensitive to mechanical distortion. Damage to visceral pleura rarely elicits pain, yet its injury profoundly impairs lung function by compromising compliance.
“Damage to the visceral layer disrupts pulmonary mechanics without warning,” notes Dr. Rajiv Nair, a thoracic surgeon specializing in pleural surgery. “The parietal layer painfully announces itself; the visceral torments silently, yet critically.”
This disparity shapes diagnostic approaches: persistent chest pain demands parietal pleura investigation, while unexplained restrictive lung patterns point to visceral involvement.
Imaging and clinical correlation remain essential, as symptoms can overlap and mimic other thoracic conditions.
Clinical Implications: From Pneumothorax to Pleural Effusion
The functional roles of visceral and parietal pleura directly influence common pathologies. Take pleural effusion—accumulation of fluid in the pleural space—and its impact on each layer.When parietal pleura ruptures, fluid enters a confined space with immediate consequences: loss of negative pressure leads to lung collapse, restricted movement, and hypoxia. In such cases, the parietal layer’s integrity determines fluid dynamics and therapeutic urgency. By contrast, visceral pleural involvement—such as fibrosis or calcification—alters lung elasticity permanently, reducing expansion capacity and increasing risk of chronic obstructive changes over time.
Pneumothorax illustrates this duality: air leaks into the pleural space through parietal breach, collapsing lung tissue; yet over time, fibrotic changes to the visceral surface further restrict function. In mediastinal shift, displacement of visceral pleura relative to parietal structures distorts thoracic anatomy, altering both respiratory mechanics and blood flow. Surgical interventions—from thoracentesis to pleurodesis—target either layer based on pathology and desired outcome, underscoring their distinct therapeutic footprints.
Diagnosis increasingly relies on modalities that differentiate visceral and parietal states: ultrasound highlights pleural line discontinuity in parietal damage, while CT reveals visceral thickening or calcification not evident on conventional imaging. Each layer thus occupies a unique diagnostic niche, guiding precise clinical decisions.
Biomechanical Compatibility and the Significance of Appoprotein Lining
Beyond sensation, biomechanical compatibility defines visceral and parietal pleura performance.The lungs’ visceral layer secretes a thin hydrophilic layer—rich in arachnoid membranes—facilitating fluid exchange and minimizing friction during respiration. This “aqueous lubrication,” as researchers term it, is absent in parietal pleura, which interfaces with stomatal tissue and connective matrices rich in collagen. This distinction preserves pleural gliding, critical for martial breathing mechanics.
When this delicate balance falters—such as in mesothelioma, an aggressive malignancy typically originating on visceral pleura—the invasive tumor disrupts normal biomechanics, inducing fibrosis and adhesions. Parenchymal damage compromises the lung’s intrinsic elasticity, and visceral invasion reprograms local immune responses, fueling progressive inflammation and effusion. Conversely, parietal pleural injury often initiates reactive mesothelial proliferation—fissuring, thickening, scarring—altering normal barrier function and predisposing to recurrent effusions.
Such distinct biomechanical roles elucidate why clinical management diverges: parietal pleura often managed symptomatically—draining effusions, treating inflammation—while visceral-involving diseases demand aggressive interventions like extrapleural pneumonectomy or bilateral pleurectomy to restore plastic function and prevent permanent lung loss.
Pathophysiological Responses and Disease Progression
At the cellular level, reactive responses differ sharply. Parietal pleura upon injury mounts a rapid fibrotic response, attempting repair through fibroblast activation and collagen deposition.This scarring is protective but may restrict lung expansion long-term. Visceral pleura, by contrast, exhibits minimal fibrosis upon injury but is highly sensitive to ischemia or infection, triggering acute inflammatory cascades that can progress to irreversible damage if untreated. Pulmonary interstitial diseases further highlight these divergent pathways.
In idiopathic pulmonary fibrosis, parietal layers thicken chronically; in diffuse pleural involvement, visceral pleura undergoes rapid, hostile remodeling that directly impairs gas exchange. Ongoing research explores targeted therapies—anti-fibrotics for parietal processes, immunomodulatory agents for visceral inflammation—emphasizing the need for layer-specific treatment strategies.
Clinical trials increasingly test interventions calibrated to pleural layer specificity, underscoring the biological uniqueness that defines each membrane’s role in health and disease.
Therapeutic Targeting and Surgical Considerations
Surgical approach hinges on layer identification: video-assisted thoracoscopic procedures distinguish parietal adhesions from visceral invasion sites, guiding resection extent and postoperative recovery. Visualization tools—fluoroscopy, intraoperative ultrasound, and near-infrared fluorescence imaging—now enable real-time mapping of pleural integrity, minimizing inadvertent damage. Beyond surgery, pleurodesis—intentional adhesion between layers to obliterate space—targeted parietal pleura prevents recurrent effusions but risks compromising visceral layers essential to lung function.Emerging techniques like fibrin glue application or zeolite particles offer layer-selective outcomes, reflecting advances in precision pleural medicine.
These innovations underscore how nuanced understanding of visceral versus parietal anatomy drives progress: preserving visceral function while managing parietal instability represents the frontier of pleural care.
Biomechanical Factors and Long-Term Functional Outcomes
Long-term respiratory competence depends on maintaining the delicate balance between these layers.Chronic parietal pleural thickening reduces thoracic compliance, mimicking restrictive lung disease. Meanwhile, progressive visceral pleural fibrosis curbs lung compliance evolutionarily, impairing exercise tolerance and escalating hypoxia risk. Patient outcomes thus hinge on early detection and tailored management that addresses both layers’ mechanical roles.
Pulmonary rehabilitation strategies increasingly incorporate pleural function assessment, adapting exercise regimens to restore lung mobility without overloading vulnerable pleural interfaces. Monitoring visceral and parietal responsiveness informs prognosis, especially in degenerative or oncologic settings.
Translational Research and Future Directions
Translational studies probe deeper into pleural biology, exploring stem cell therapies to regenerate damaged parietal linings and gene-targeted approaches to modulate vis Cer elliptic pleural inflammation.Animal models demonstrate that repairing visceral pleura restores normal gas exchange, while stabilizing parietal integrity prevents airspace collapse. Emerging imaging biomarkers promise earlier, layer-specific diagnosis—detecting microfractures in visceral pleura before fibrosis develops, or identifying parietal invaginations modeling effusion risk. Such tools may revolutionize preventive care, shifting focus from symptom management to structural preservation.
As science unravels pleural complexity layer by layer, the visceral and parietal distinctions remain central—anchors in physiology, division in pathology, lens in treatment.
Conclusion
The interplay between visceral and parietal pleura forms the cornerstone of thoracic function, defining how lungs expand, contract, and interface with the body wall. While both prevent collision and sustain respiration, their origins, sensory profiles, biomechanical roles, and clinical responses diverge in ways with profound implications.Understanding these distinctions enables precise diagnosis, targeted intervention, and better patient outcomes—from managing pleuritic pain to reversing fibrotic change. In embracing the visceral’s silent sentinel role and the parietal’s structural vigil, medicine advances toward a more nuanced, effective frontier in lung care.


Related Post
Cristoferideas Unveils How Innovation, Creativity, and Strategy Are Rewriting Global Success in the 21st Century

Alice In Wonderland 2010 Cast And Character Guide
Honoring Comedian Yvette Wilson: A Journey Sadly Ended

Karina Garcia’s Journey From 600 Lbs to 400 Lbs: A Transformative Instagram Voyage of Health and Humanity

