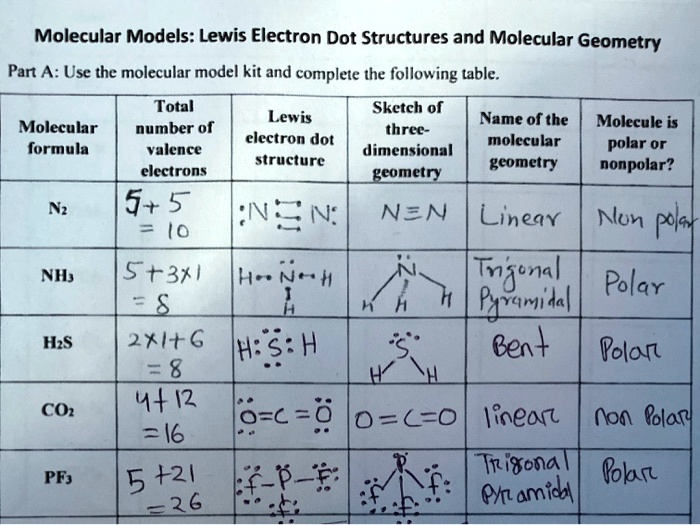
Unveiling the Geometry of BF₃: How Lewis Structure Reveals Its Electron Puzzles
Unveiling the Geometry of BF₃: How Lewis Structure Reveals Its Electron Puzzles
Boron trifluoride (BF₃) stands as a paradigmatic example in chemical bonding, revealing how electron distribution shapes molecular geometry and reactivity. Central to understanding BF₃ is the Lewis structure, a foundational tool that maps valence electrons and bonding patterns, offering deep insight into its trigonal planar form and chemical behavior. This article explores how boron’s electron-deficient nature, fluorine’s strong electronegativity, and precise orbital overlap culminate in BF₃’s characteristic structure—pred dicated by bond angles, electron repulsion, and geometric constraints.
At the core of BF₃’s structure lies a simple carbon-boron-fluorine electron-sharing model, revealed through the Lewis dot diagram. Boron, with three valence electrons, forms three covalent bonds with three fluorine atoms, using all three valence electrons to achieve a stable electron configuration. Fluorine, each possessing seven electron shells, readily accepts one electron per bond, filling its outer shell and minimizing repulsive forces.
The resulting Lewis structure displays boron at the center, triple-bonded in conceptual terms to each fluorine, though in reality a partial double-bond character dominates—a nuance critical for understanding the molecule’s reactivity. Though BF₃ lacks a formal double bond, electron density around boron remains polarized, generating intriguing chemical dynamics.
Orbital Overlap and the Trigonal Planar Framework
The trigonal planar geometry of BF₃ emerges directly from carbon boron oxidation state and sp² hybridization, confirmed visually by the Lewis structure’s planar arrangement.Boron achieves optimal electron pairing through sp² hybrid orbitals, which align at 120° angles, minimizing electron pair repulsion as predicted by VSEPR (Valence Shell Electron Pair Repulsion) theory. Fluorine atoms, each contributing six valence electrons, occupy hybrid sp² orbitals and donate lone pairs into empty p orbitals, forming sigma bonds with boron. This precise orbital geometry results in molecular orbital symmetry that stabilizes BF₃ in its flat, symmetrical form—a hallmark of efficient electron sharing and charge distribution.
Each B–F bond reflects localized electron density concentrated in sigma (σ) bonds, with no contribution from π bonds due to boron’s empty p orbital. This absence of back-donation explains BF₃’s status as a Lewis acid: electron-deficient boron readily accepts electron pairs, enabling diverse acid-base and catalytic interactions. "The Lewis structure is not merely a sketch—it’s a predictive map of bonding capability and molecular identity," notes Dr.
Elena Martinez, chemical structure expert at the Institute for Molecular Geometry. "BF₃’s planar arrangement arises naturally from electron count, hybrid orbital orientation, and spatial efficiency.”
The Role of Electron Count and Incomplete Octets
Boron’s three valence electrons create an electron configuration identical to helium, leaving it electron-deficient. In BF₃, boron forms three bonds but retains an open p orbital—resulting in a formal positive charge and incomplete octet.This electron deficiency drives BF₃’s high reactivity, particularly its ability to act as a strong electrophile. The Lewis structure illustrates this open-shell condition: polygonal skeleton with three vertices and three $\sigma$ bonds, explicitly showing "missing" electrons available for interaction. Unlike species with expanded octets, BF₃ resists such deviations, preserving structural integrity through minimal electron redistribution.
Symmetry, Polarizability, and Intermolecular Interactions
The symmetry of BF₃’s trigonal plane produces a net dipole moment of zero—a consequence of vector cancellation between the three cohesive B–F bonds. This molecular symmetry limits dipole-induced alignment but enhances lattice stability in the solid state, where consistent spatial orientation maximizes lattice energy through favorable steric and electrostatic compatibility. Fluorine’s strong electronegativity amplifies localized partial positive charges on boron, making BF₃ prone to nucleophilic attack.This polarizability, rooted in the clear electron geometry from Lewis structures, underpins its utility in Boron-Fluorine chemistry and as a catalyst intermediate.
Visually, the BF₃ Lewis structure—simple yet powerful—encapsulates a wealth of bonding principles: hybridization, electron deficiency, bond angle geometry, and reactivity directionality. These features confirm BF₃ not as a static molecule, but as a dynamic entity shaped by quantum constraints and electron behavior.
The structure’s elegance lies in its precision: three bonds, trigonal symmetry, zero net dipole—each derived directly from electron counting and orbital overlap, verified through the clarity of the Lewis representation. Ultimately, BF₃’s story is one of elegant simplicity disguised within a framework of fundamental chemistry. From its minimalist electron dots to its balanced trigonal planarity, Lewis structure analysis reveals more than shape—it explains why BF₃ reacts, reacts the way it does, and why such behavior matters in industrial applications, materials science, and chemical synthesis.
This enduring molecule reminds us that even the most basic structures carry profound intellectual and practical weight.


Related Post

