Unlocking the Water Molecule: The Hidden Brilliance Behind Lewis Dot Representation
Unlocking the Water Molecule: The Hidden Brilliance Behind Lewis Dot Representation
Water, the foundation of life, remains one of nature’s most complex and vital substances. At its core, the Lewis dot structure of water reveals a deceptively simple yet profoundly sophisticated arrangement of electrons that governs its unique physical and chemical behavior. This tiny molecule, composed of two hydrogen atoms covalently bonded to a central oxygen atom, exemplifies how microscopic electron interactions drive macroscopic phenomena—from fluidity and hydrogen bonding to its role in sustaining biological systems.
Understanding the Lewis dot representation of water is not just academic; it is key to unlocking deeper insights into molecular dynamics, reactivity, and the very essence of life’s chemistry.
The Lewis Dot Structure of Water: Electron Architecture at a Glance
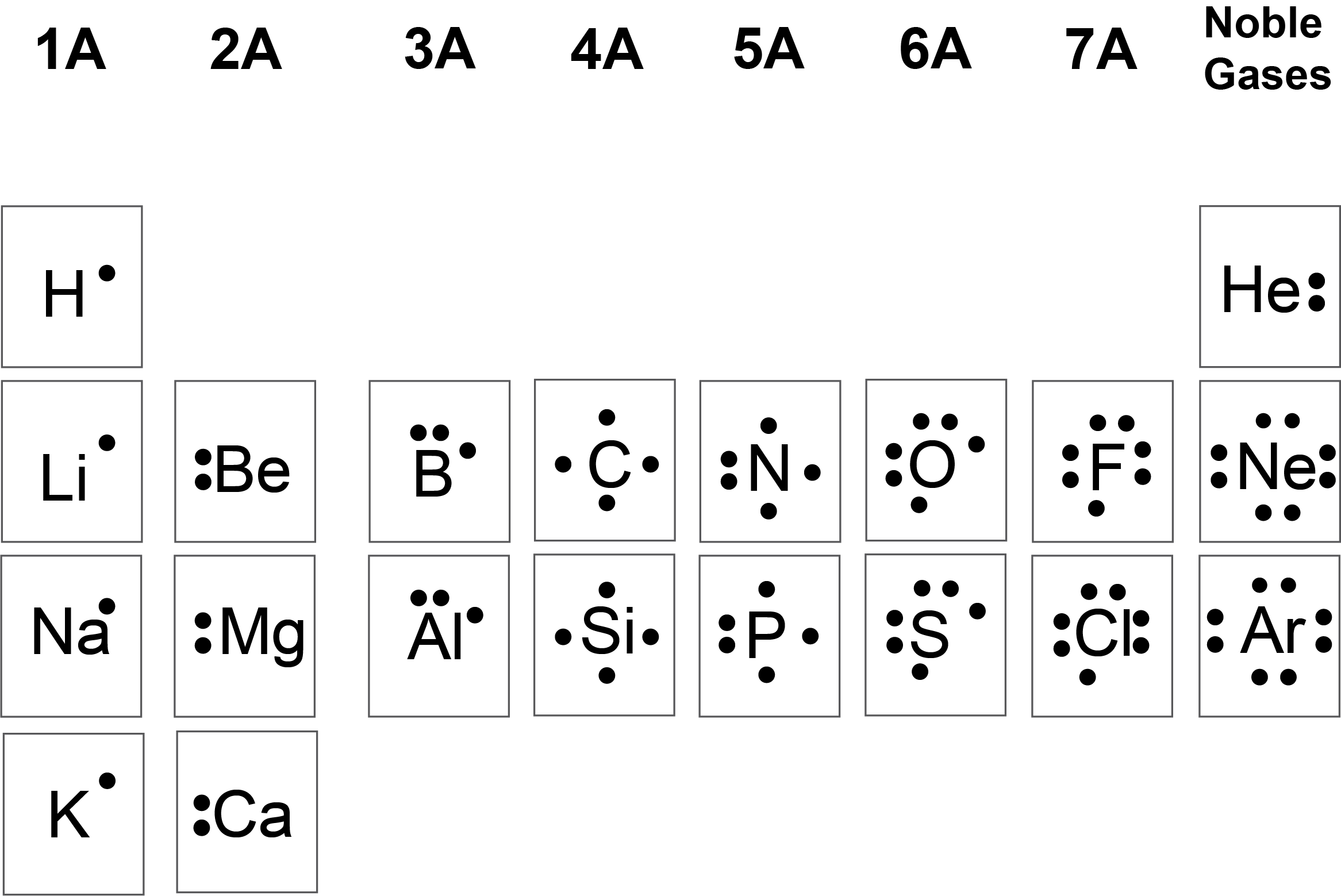
The Lewis dot structure of water 단순 yet telling: an oxygen atom in the center, surrounded by two hydrogen atoms, with shared electron pairs forming two strong covalent bonds. The oxygen atom carries six valence electrons—two of which it donates to each hydrogen, placing it elegant in its tour.Each hydrogen contributes one electron to the shared pair, resulting in a total of eight electrons around oxygen (extension rules beyond the octet in resonance structures) and two around each hydrogen. The molecule adopts a bent geometry with a precise bond angle of approximately 104.5 degrees, shaped by electron pair repulsion—a phenomenon formalized in VSEPR theory. Each hydrogen atom forms a sigma bond via a single pair shared between O and H, enabling compact, stable bonding.
“The structure may appear straightforward,” observes Dr. Elena Rodriguez, physical chemist at Princeton University, “but it is this precise electron arrangement that powers water’s unparalleled versatility—from dissolving essential nutrients to participating in biochemical catalysis.” The lone pairs on oxygen, critical to water’s polarity and hydrogen bonding capacity, remain a focal point in explaining its anomalous properties.
Electron Distribution and Polarity: Why Water Behaves Uniquely
While the Lewis structure depicts symmetric sharing, water’s true magic lies in its electron distribution.Oxygen, more electronegative than hydrogen, exerts a strong pull on the shared electron pairs, creating a permanent dipole moment. This polarity—hydrogen bearing partial positive charges, oxygen partial negative—drives water’s exceptional solvent capabilities. “The uneven electron density isn’t just a detail; it’s the origin of water’s biological power,” explains Dr.
Marcus Lin, an expert in molecular chemistry from MIT. “It explains why water is the universal medium for life’s reactions—enabling ion transport, enzyme function, and hydration of biomolecules.” Polarity manifests in measurable dielectric properties, low surface tension relative to other polarants, and strong cohesive energy. These traits stem directly from the distributed electron density revealed in the Lewis dot model.
Without this electron architecture, water’s cohesion would collapse, and its role as a biochemical sweetspot would vanish. The structure thus serves as a blueprint for understanding solvation, hydration shells, and the thermodynamics of aqueous systems.
The Role of Bond Angles and Electron Pairs in Molecular Style The 104.5° angle is more than a geometric footnote—it reflects electron geometry shaped by both bonding and lone pairs.
According to VSEPR theory, oxygen’s hybrid orbital arrangement minimizes repulsion among electron domains, favoring a bent configuration. Each lone pair occupies more space than bonding pairs, compressing the H–O–H angle slightly below a perfect tetrahedral 109.5°. This small but significant angle enhances polarity and enables water’s persistent intermolecular hydrogen bonds, a hallmark of liquid and solid-phase water.
Parallel systems—such as methane (CH₄) with its 109.5° tetrahedral profile—contrast with water, highlighting how subtle changes in electron pair geometry reshape molecular identity. “The bent geometry of water is a masterstroke of evolutionary efficiency,” remarks Dr. Lin.
“It maximizes hydrogen bonding while maintaining fluidity—critical for cellular transport and nutrient distribution.”
Resonance and Stability: The Delocalized Electron Tale Contrary to static depictions, modern value positions the Lewis dot structure within a resonance framework. While simplified representations show two equivalent O–H bonds, true electron distribution involves partial delocalization across the molecule, stabilized by conjugation between oxygen’s lone pairs and hydrogen bonding orbitals. This dynamic electron flow enhances chemical resilience, allowing water to act as both donor and acceptor in countless reactions.
Resonance stabilizes the molecule by dispersing charge density, reducing electrostatic strain and fortifying intermolecular interactions. The result is a remarkably cohesive substance with consistent physical properties across temperatures and pressures—qualities indispensable to planetary habitability.
Real-World Impacts: From Biology to Climate Science
The Lewis dot configuration of water underpins phenomena critical to life and Earth systems.Hydrogen bonding, enabled by polar oxygen lone pairs and shared electron domains, gives water a high heat capacity—regulating temperatures in organisms and oceans alike. Cohesive adhesion powers capillary action in plants, lifting water from roots to leaves in trees 100 meters tall. Even as a universal solvent, water’s polar nature dissolves minerals, gases, and biomolecules, making it the solvent of life.
On a planetary scale, water’s helicpectron architecture drives the hydrological cycle, influences atmospheric chemistry, and moderates global climate patterns. “Every drop of water reflects a quantum electron dance,” notes Dr. Rodriguez.
“From a single H₂O molecule, we see a microcosm of chemical logic that sustains ecosystems and climate alike.”
The Lewis Dot Structure: A Cornerstone of Water’s Identity
The Lewis dot representation of water is far more than a classroom sketch—it is a scientific narrative etched in electron distribution. It reveals how two hydrogen atoms, each craving stability, align precisely with oxygen’s electron-rich center to form bonds that define water’s dual nature: liquid at room temp, solid as ice, and polar to unparalleled. This structure unlocks explanations for hydrogen bonding, solubility, and dynamic hydrogen networks essential to biochemistry and earth science.By decoding the electron makeup, scientists illuminate water’s role in life, technology, and planetary systems. As Dr. Lin asserts, “Understanding the Lewis dot of water means more than drawing dots and lines—it means grasping the heartbeat of chemistry that sustains everything.”



Related Post

Nilay Patel: The Voice Behind Transformative Tech Podcasts, Wife, and Life in Balance
Wissports: Unpacking the Digital Transformation of College Athletics
80 Robux Purchases: Unlocking Power, Status, and Virtual Identity in Roblox

