Unlocking the Secrets of Sodium: Decoding the Orbital Diagram Behind Its Magnetic Dance
Unlocking the Secrets of Sodium: Decoding the Orbital Diagram Behind Its Magnetic Dance
Pulsing with invisible energy yet vital to daily life, sodium’s behavior at the atomic level reveals profound insights into matter, magnetism, and material science. Central to understanding sodium’s complex electron configuration and its striking physical properties is the orbital diagram—a blueprint that maps how electrons fill quantum states. The orbital diagram of atomic sodium, particularly its dynamic electron arrangement in the outer 3s orbital, unlocks why sodium is reactive, conductive, and responsible for defining signatures from streetlights to biological signals.
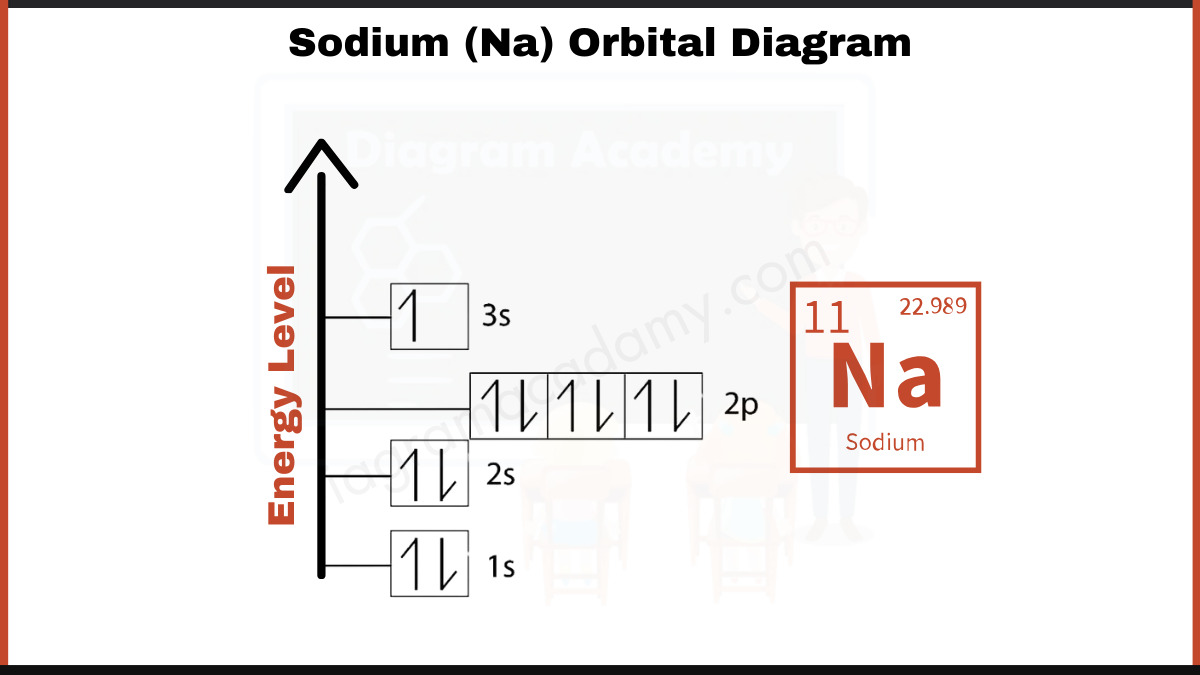
This deep dive illuminates how sodium’s electron configuration governs its chemical behavior and technological applications, guided by a precise orbital diagram that reveals the dance of electrons shaping our world. The atomic structure of sodium (Na) places it in Group 1 of the periodic table, with an electron configuration of 1s² 2s² 2p⁶ 3s¹. This single valence electron in the 3s orbital is the key to sodium’s reactivity and its pronounced response to external stimuli.
The orbital diagram captures this electron’s quantum state, illustrating how it occupies the largest electron shell—the 3s orbital—whose spherical symmetry allows for low-energy stabilization but high mobility. Unlike inner shell electrons tightly bound to the nucleus, sodium’s 3s electron exists in a higher-energy frontier, easily yielding its energy during ionization. The orbital representation of sodium reveals critical insights into its electronic shell structure.
With two electrons in the first shell (1s²), six in the second (2s² and 2p⁶), and just one unpaired electron in the 3s orbital, sodium’s configuration achieves a stable but accessible electron arrangement. “This lone outer s electron makes sodium highly reactive, eager to shed one and achieve a noble gas configuration,” explains Dr. Elena Marquez, a quantum physicist specializing in alkali metals.
“It’s this very vulnerability that powers sodium’s signature flame—bright yellow, a hallmark in flames from candles to fireworks.” The orbital diagram visually emphasizes not only the number but the spatial occupation of electrons. While the 2p subshell hosts a full shell with six electrons, the 3s orbital remains singly occupied, highlighting sodium’s position in the periodic table as a metal where electron mobility defines its hallmark conductivity. “Each orbital follows strict quantum rules,” notes Dr.
Marquez. “The 3s orbital can hold up to two electrons with opposite spins, but here it holds one—what we call a ‘half-filled’ state within a larger filled shell. This relative emptiness drives sodium’s ionization energy and reactivity.” Understanding sodium’s orbital architecture extends beyond static electron count—it reveals dynamic behavior.
When heated, sodium rapidly loses its 3s electron, forming Na⁺ ions that pair with anions in ionic compounds or cluster into metallic lattices defined by delocalized electrons. This quantum behavior underpins sodium’s role in biological nerve signaling, battery electrolytes, and industrial processes. “The orbital diagram bridges theory and application,” says Marquez.
“It’s not just a drawing—it’s a predictive tool showing how electrons behave under real conditions, from laboratory plasma to biological ion channels.” Orbital diagrams for sodium, while seemingly abstract, are powerful visual narratives of atomic identity. They explain why sodium emits light at 589 nm—responsible for its iconic spectral line—because electron transitions trace back to this single, energetic 3s electron. “The diagram decodes the energy gaps,” explains Marquez.
“When energy excites the unpaired 3s electron, it jumps to higher states and releases photons, generating sodium’s fierce yellow glow.” This process, fundamental to both astronomy and lighting technology, becomes intelligible through orbital visualization. Beyond visual allure, the orbital depiction informs practical innovation. In solid-state chemistry, controlling sodium’s electron mobility via orbital manipulation enables advances in sodium-ion batteries—critical for sustainable energy storage.
The diagram also guides material science, illustrating how sodium’s electronic structure influences alloy behavior and superconducting properties when combined with other elements. Ultimately, the orbital diagram of sodium is more than a technical illustration—it is a lens through which to understand reactivity, identity, and utility. Each electron’s placement reveals sodium’s core nature: a reactive, conductive, light-emitting metal whose atomic choreography shapes everyday technology and natural phenomena alike.
As quantum mechanics continues to inform material innovation, sodium’s orbital skeleton remains a cornerstone of scientific insight—quietly powerful, elegantly structured, and profoundly relevant.
In mastering sodium’s orbital diagram, science unveils the hidden logic behind one of Earth’s most influential elements—turning invisible electrons into understood forces that power flame, light, and the future of sustainable energy.



Related Post

Ijadens Thermal Printer Amin Analysis: The Best Low-Cost Solution for Scan-and-Print Needs?

Spiralling Spirit Locker Room A Comprehensive Guide To Boost Team Morale and Performance Unlocking the Power of the Spiralling Journey

Unlock Elite Savings with CreaviteCo Discord Profile Coupon Code – Here’s What You Need to Know

