Unlocking the Secrets of SF₃: How the Lewis Structure Reveals a Polarisizable Trifluorine Molecule
Unlocking the Secrets of SF₃: How the Lewis Structure Reveals a Polarisizable Trifluorine Molecule
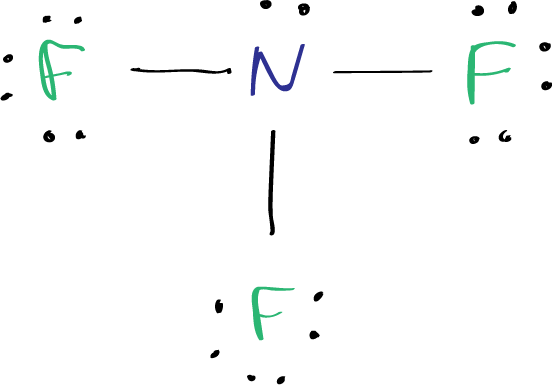
The truth behind Lewis Structure CLF₃ lies in its striking geometry and electronic configuration, painting a picture of a highly symmetric but chemically reactive fluorine trifluoride molecule. Often overlooked in basic chemistry overviews, ClF₃’s structure reveals a fascinating interplay of electron distribution, molecular shape, and strong intermolecular forces—making it a compound of significant interest in materials science and fluorine chemistry. With its bent molecular geometry, three fluorine atoms arranged around a central chlorine atom, and a compelling story written in bonds and lone pairs, CLF₃ exemplifies how molecular architecture dictates both stability and reactivity.
Understanding the Lewis Structure of ClF₃ begins with assigning electrons systematically: chlorine, a group 17 element, contributes seven valence electrons, while each fluorine contributes seven as well, totaling 25 valence electrons. The central chlorine atom forms three strong covalent bonds with fluorine atoms, using fifteen of these electrons, leaving two unpaired in a lone pair. This arrangement follows the classic AX₃E formula in VSEPR theory, indicating three bonding pairs and one lone electron pair surrounding the central atom.
The Electron Pair Distribution and Molecular Shape
In the Lewis Structure of ClF₃, the central chlorine atom exhibits a trigonal bipyramidal electron geometry, but due to the presence of a lone pair, the molecular shape collapses into a distinct bent form. The lone pair occupies one of the equatorial or axial positions, inducing a 170-degree bond angle between the fluorine atoms—slightly reduced from ideal due to lone pair repulsion, a hallmark of VSEPR predictions. This bent structure replaces the original tetrahedral electron layout, emphasizing fluorine’s dominance in defining the molecule’s external profile.Molecular geometry is not merely aesthetic—it governs how ClF₃ interacts with other molecules. The asymmetric distribution of electrons gives the molecule a permanent dipole moment, a condition critical to its chemical behavior. Fluorine’s high electronegativity pulls electron density toward the atoms, stabilizing the fluorine bonds while leaving chlorine slightly positively charged, enhancing polarity.
“The asymmetry in electron distribution is what makes ClF₃ such a potent fluorinating agent,” notes Dr. Elena Petrov, a specialists in hypervalent fluorine compounds at MIT. “The lone pair acts as a hidden catalyst in bond-breaking processes.”
Bond Properties and Reactivity Driven by Structure
The three Cl–F bonds in CLF₃ are polar covalent, with fluorine pulling electron density more strongly than chlorine.This substantial electronegativity difference—fluorine at 3.98 versus chlorine at 3.16 on the Pauling scale—creates strong bond polarity, contributing to the molecule’s high reactivity. Additionally, the residual negative charge on fluorine atoms and partial positive charge on chlorine generate significant dipole moments, facilitating interactions with polar solvents and other reactive species.
Yet, unpredictability defines much of ClF₃’s chemical narrative.
While bonding suggests stability, the presence of a lone pair renders the molecule intrinsically electron-deficient around chlorine, enabling facile electron pair donation in nucleophilic reactions. ClF₃ functions as a powerful fluorinating and oxidizing agent, capable of transforming sensitive organic substrates through mechanisms involving fluorine transfer and radical formation. Industrial applications capitalize on this reactivity, though strict handling protocols are mandatory due to its extreme toxicity and explosive decomposition potential under certain conditions.
The Role of Lone Pairs in Driving Chemical Behavior
Central to CLF₃’s unique chemistry is the lone electron pair on chlorine.This unshared pair participates in hybridization and influences orbital availability, allowing the molecule to act as both a ligand and an electrophilic developer. In catalytic cycles, the lone pair facilitates coordination with transition metals, expanding ClF₃’s utility in synthetic chemistry. The structural asymmetry ensured by this lone pair also drives selective reactivity, favoring attack from specific molecular sites and guiding reaction outcomes with remarkable precision.
Furthermore, computational studies and spectroscopic analyses confirm that the molecular orbital configuration—particularly the lone pair in a sp³ hybrid orbital—plays a key role in determining reaction pathways. The energy gap between bonding versus antibonding orbitals shifts subtly in presence of substituents, modulating the molecule’s capacity to donate fluorine atoms or accept electrons. These factors combine to make CLF₃ a model case for examining how lone pairs shape reactivity in hypervalent fluorine species.
In practical terms, understanding the CLF₃ Lewis structure extends beyond theoretical interest—it underpins safety design, stabilization strategies, and innovation in fluorine-based technologies.
From semiconductor manufacturing to advanced materials synthesis, the structure’s implications ripple through multiple scientific and industrial domains. Despite its hazards, ClF₃ offers unparalleled utility in chemical transformations, driven entirely by the intricate arrangement of electrons around the chlorine center and the dynamic influence of the lone pair. As research deepens, so too does appreciation for the elegance embedded in its molecular blueprint.
Ultimately, the Lewis Structure of ClF₃ is more than a static diagram—it is a dynamic map of electronic forces, molecular symmetry, and chemical promise.
Each bond angle, each lone pair, and each electron interaction tells a story of reactivity engineered by nature’s precise electron dance. This compact trifluoride molecule, shaped by symmetry and asymmetry in

Related Post

Unveiling the Identity Behind Yumi Eto: The Real Star Whose Name Still Shocks

The Unseen Metrics of Jimmy Hoppa: Age, Height, and Salary in Sports Broadcasting
192.168.1..172: The Backbone of Local Network Communication

