Unlocking the Secrets of Molar Mass: The Molecular Weight That Defines Chemistry
Unlocking the Secrets of Molar Mass: The Molecular Weight That Defines Chemistry
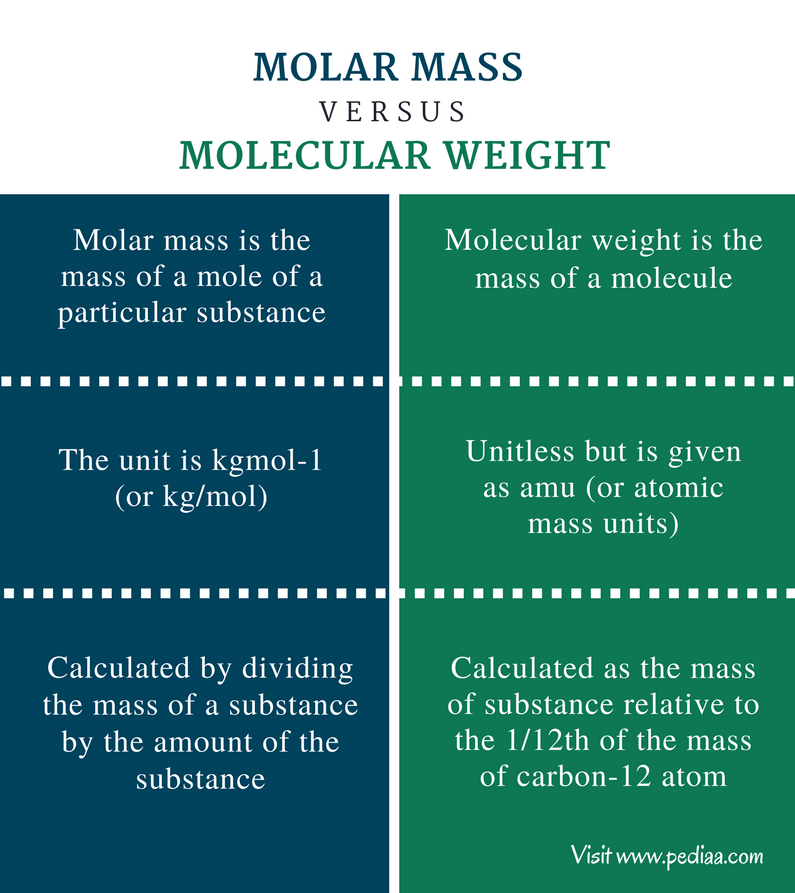
Na Molar Mass, though often overlooked, stands as a cornerstone in chemistry—bridging the atomic scale to the macroscopic world. Defined as the mass of one mole of a substance measured in grams per mole (g/mol), molar mass is the invisible metric that unlocks stoichiometry, enables precise chemical calculations, and governs molecular behavior across pharmaceuticals, materials science, and environmental chemistry. It is not merely a numerical value but a fundamental parameter that dictates how chemicals interact, react, and exist in nature.
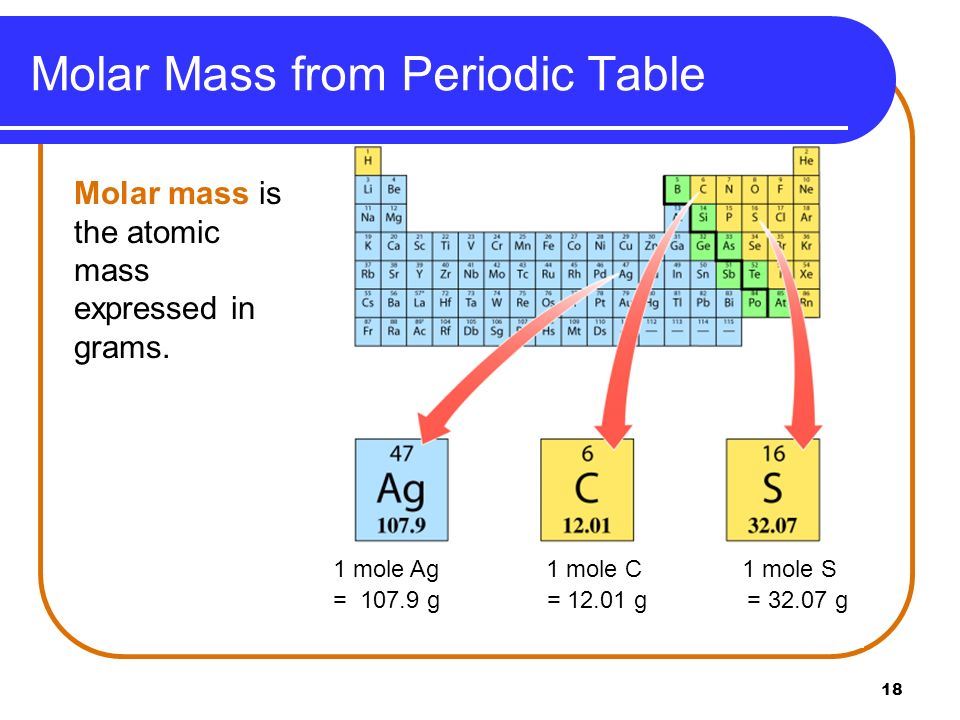
At its core, molar mass represents the aggregate mass of atoms composing a single mole—6.022×10²³ molecules or atoms—as prescribed by Avogadro’s number.
For a single element like carbon, with an atomic mass of 12.01 atomic mass units (amu), the molar mass is exactly 12.01 g/mol. For compounds, it becomes a composite value: water (H₂O) has a molar mass of 18.015 g/mol, derived from two hydrogen atoms (1.008 g/mol each) and one oxygen (16.00 g/mol), totaling 18.015 g/mol. This precise summation of atomic weights forms the basis for quantitative chemical analysis.
Why Molar Mass Matters in Stoichiometry and Lab Precision
In laboratory settings, molar mass serves as the linchpin of stoichiometric calculations—the quantitative relationships governing chemical reactions.
Without accurate molar masses, chemists would lack the tools to convert grams to moles, a conversion essential for predicting reactant consumption and product formation. The formula moles = mass (g) ÷ molar mass (g/mol) is foundational to balanced chemical equations and lab experiments.
Consider the synthesis of aspirin (acetylsalicylic acid), C₉H₈O₄: its molar mass is approximately 180.16 g/mol. To prepare 0.5 moles of aspirin requiring 4.5 grams, a researcher relies on molar mass to ensure accuracy.
Incorrect mass inputs—even by a gram—can lead to failed reactions or impurities, underscoring the necessity of precise measurement. Modern analytical instruments, from balances to titrants, calibrate tightly around molar mass standards to maintain reproducibility.
The Role of Molar Mass in Pharmaceutical and Industrial Applications
Beyond the lab, molar mass governs drug development and industrial manufacturing. In pharmaceuticals, dosage precision hinges on accurate molar mass calculations.
A medication’s therapeutic efficacy depends on delivering exact molar quantities; deviations risk underdosing or toxicity. Regulatory bodies like the FDA mandate strict adherence to molar mass standards in drug formulation to ensure safety and uniformity.
Industrially, molar mass drives quality control in polymer production. Polymers exhibit properties directly tied to molecular weight—length of chain segments, chain branching, and overall molar mass distribution.
High-density polyethylene (HDPE), with a typical molar mass of 200,000–200,000 g/mol, demonstrates enhanced strength and durability compared to low-density variants, illustrating how molar parameters direct material performance. Characterization techniques, such as gel permeation chromatography (GPC), use molar mass data to verify polymer integrity before commercial use.
Measuring Molar Mass: From Theory to Calibration
Determining molar mass combines theoretical computation with experimental verification. Atomic masses, defined by the International Union of Pure and Applied Chemistry (IUPAC), are based on naturally occurring isotopic abundances—carbon-12 set to exactly 12 amu, for instance.
To compute molar mass of a compound, the sum of individual atomic masses is adjusted for average isotopic distribution.
For example: water’s molar mass is computed as (0.98 × 1.008) + (0.02 × 16.00) = 18.015 g/mol—mirroring its natural isotopic ratios. Mass spectrometry and AVOGA spectrometry provide direct measurements of molecular mass, validating theoretical predictions and refining reference databases. Such precision underpins analytical chemistry, where trace analysis demands rigorous molar mass precision.
Real-World Examples: From Carbon Dioxide to Catalytic Converters
Consider carbon dioxide (CO₂), a molecule central to climate science and industry.
Its molar mass is 44.01 g/mol, calculated as 12.01 + (2 × 16.00). This value informs atmospheric modeling, greenhouse gas accounting, and carbon capture technologies. In environmental monitoring, accurate molar mass allows scientists to quantify CO₂ concentrations with high specificity, critical for policy and mitigation strategies.
Even catalytic converters in automobiles rely on molar mass principles.
Platinum-based catalysts accelerate reactions converting toxic CO and NOₓ into less harmful CO₂ and N₂. The stoichiometry of these reactions depends on precise molar ratios of reactants—enabled by accurate molar mass data—to maximize conversion efficiency under dynamic engine conditions.
Educational Tools and Advanced Applications
In academic settings, molar mass serves as a gateway to deeper chemical understanding. Students use it to explore conceptual links between atomic structure and macroscopic properties—from molar volume of gases at STP (22.4 L/mol) to molarity in solution chemistry (moles per liter).
These foundational lessons translate into advanced techniques like reaction kinetics, where molar concentrations dictate rate equations and activation dynamics.
In materials science, tailoring molar mass optimizes properties. Smooth polymers with controlled molar mass achieve superior mechanical strength; aerogels with precise molecular architectures enable record-breaking insulation. This level of control rests on mastering molar mass principles—from synthesis planning to post-fabrication analysis.
The consistent use of molar mass across disciplines—from analytical labs to industrial production—cements its role as a universal metric.
It is not a mere number but a vital language of chemistry, translating atomic-scale phenomena into real-world applications that shape technology, health, and sustainability.
Understanding Na Molar Mass in its full depth reveals a quiet yet profound influence on the chemical world. It empowers scientists to navigate complexity with precision, ensuring that every reaction—from a lab bench to a catalytic converter—unfolds as intended. In the arena of molecular science, molar mass remains the silent architect of control, consistency, and innovation.


Related Post
Unveiling the Digital Web of Fmovies.Com: A Comprehensive Dive

Bob Griese’s Net Worth: From Pro Football Legend to Enduring Legacy in Business and Philanthropy
Behind the Shadows: 10 Disturbing Details Hidden in the DD Blanchard Crime Scene Photography

