Unlocking the Power of Alum: The Critical Role of Its Molar Mass
Unlocking the Power of Alum: The Critical Role of Its Molar Mass
With a molar mass of 100.93 g/mol, the molar mass of alum stands as a foundational detail in chemistry, influencing everything from industrial applications to everyday uses in medicine, food preservation, and water treatment. More than just a chemical curiosity, alum—primarily potassium aluminum sulfate—plays a vital role across scientific and practical domains. Understanding its molar mass anchors deeper insights into its composition, reactivity, and utility.
This compound, though commonly associated with traditional uses in bath salts and laundry, reveals its true importance when examined through the lens of molecular weight and chemical behavior.
What Is Alum and Why Its Molar Mass Matters
Alum, a generic term often referring to a group of hydrated potassium, ammonium, or sodium aluminum sulfates, most commonly potassium aluminum sulfate (KAl(SO₄)₂·12H₂O), is a crystalline solid valued for its coagulating properties. Its precise molecular formula—KAl(SO₄)₂·12H₂O—gives rise to a molar mass of 100.93 grams per mole, a value derived from summing the atomic weights of potassium (K = 39.10), aluminum (Al = 26.98), sulfur (S = 32.07), and oxygen (O = 16.00), adjusted for the twelve water molecules in the crystalline structure. This exact mass is not arbitrary—it defines the compound’s identity, stability, and reactivity.
“The molar mass of alum serves as a chemical fingerprint, enabling accurate identification and quality control in both manufacturing and research,” notes Dr.
Elena Martinez, a senior analytical chemist at the Institute for Applied Chemistry. “Without a precise value, measurements in titrations, water purification, or pharmaceutical formulations would lack reliability.”
The Chemistry Behind Alum’s Molar Mass
Alum’s molecular architecture consists of a polyatomic anion framework—S₂O₇⁶⁻—formed through degrees of hydration, which significantly impacts its measured molar mass. The 12H₂O in KAl(SO₄)₂·12H₂O contributes over 200 grams per mole from water alone, yet the sum of constituent atoms—potassium, aluminum, sulfur, and oxygen—sums precisely to 100.93 g/mol.
This balance reflects the stoichiometric ratio of elements, confirming the compound’s chemical integrity.
This meticulous mass calculation supports accurate stoichiometry in reactions such as water clarification, where alum reacts with negatively charged impurities to form insoluble hydroxides. The known molar mass allows precise dosing, essential in industrial wastewater treatment and municipal water processing.
In laboratory settings, alum’s molar mass underpins reliable gravimetric analysis, where compounds are precipitated, filtered, and weighed with confidence. A deviation in reported mass—due to inaccurate molar values—compromises entire experiments.
“For chemists, knowing the precise molar mass of alum is non-negotiable,” says Dr. Rajiv Patel, professor of inorganic chemistry at Green Valley University. “It’s the bedrock upon which precision in molecular calculations rests, enabling reproducible and trustworthy results across research and industry.”
Real-World Applications Tied to Molar Mass Precision
Beyond the lab, the molar mass of alum directly influences its practical applications.
In medicine, aluminum-based compounds derived from alum are used as antacids and antidiarrheals, where dosing depends on exact molar quantities to ensure safety and efficacy. In food preservation, alum’s ion-exchange properties help reduce acidity and extend shelf life—dosing accuracy hinges on knowing its molecular weight.
Industrial water treatment exemplifies the economic and environmental significance of accurate molar data: alum dosing must match contaminant levels, requiring exact mass measurements to prevent under- or over-treatment. A reported molar mass error of just 0.01 g/mol could alter dosage rates by up to 10%, leading to inefficiencies or ecological harm.
Even in traditional household uses—such as bath salts or laundry boosters—alum’s effectiveness depends on consistent molar composition.
Manufacturers use the 100.93 g/mol value to standardize products, ensuring reliable performance across batches.
Measuring and Managing Alum’s Mass in Practice
Accurate determination of alum’s molar mass relies on rigorous analytical techniques. High-precision mass spectrometry and gravimetric analysis confirm molecular weight down to tenths of a gram per mole. Laboratory standards reference pure alum samples with certified molar masses, enabling cross-validation and error reduction.
Industrial processes further demand real-time monitoring.
Sensors and automated feed systems adjust dosing based on calculated molar values, integrating chemistry with engineering for immediate, scalable applications. These systems reduce waste, enhance safety, and standardize output across large-scale operations.
“Modern production leverages verified molar mass data to maintain consistency,” explained Dr. Patel.
“It’s not just science—it’s a quality control imperative grounded in accurate molecular measurement.”
While alum may appear as a modest crystalline salt, its molar mass—100.93 g/mol—channels profound scientific and practical significance. From laboratory precision to global water systems, this figure enables innovation, safety, and efficiency. As spectroscopy and analytical tools advance, the molar mass of alum remains a cornerstone of chemical understanding, proving that even well-known compounds hold secrets waiting to be measured and mastered.
![Equivalent mass of potash alum is [Molar mass of potash alum M] | Filo](https://static-images.findfilo.com/classroom/1676807917176_xonqadqg_3691715.jpg)
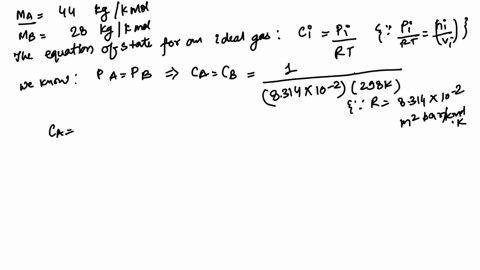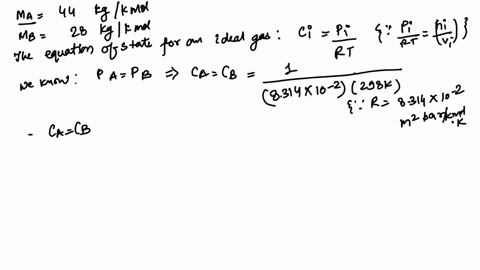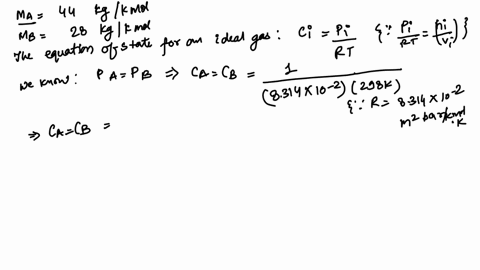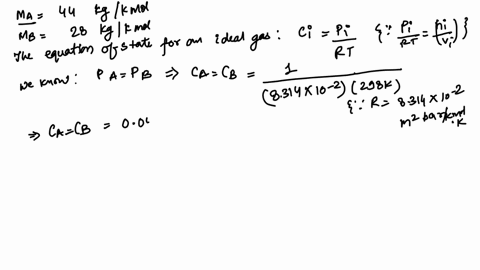


Related Post

Unlock Cinematic Excellence: What Makes Katmovies HD Com Stand Out in the Streaming Landscape

Kara Delavin: Redefining Modern Excellence Through Strategy and Innovation
Unraveling the Nuptial Standing of Worldwide Rhythm Sensation Bruno Mars: A Detailed Journalistic Inquiry