Unlocking the Force of Nature: How Boyle’s Law Graph Reveals the Hidden Pressure of Air
Unlocking the Force of Nature: How Boyle’s Law Graph Reveals the Hidden Pressure of Air
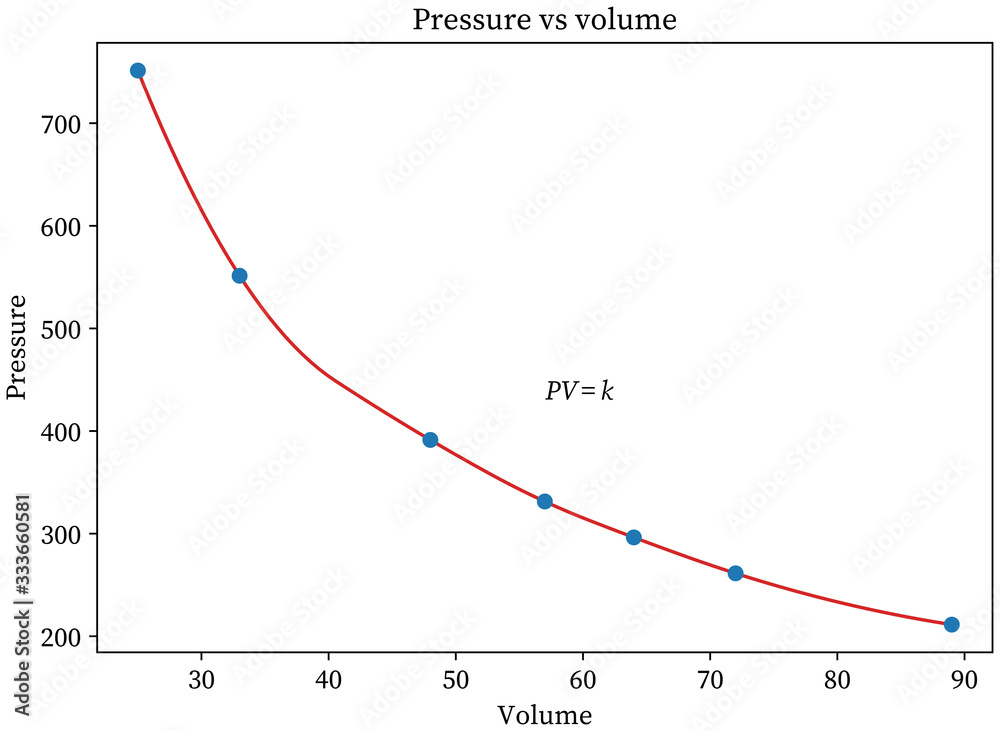
A single curve on a pressure-volume graph holds the key to understanding the invisible forces that shape everyday life—from the breath in our lungs to the operation of industrial machinery. This is Boyle’s Law in motion, a foundational principle in physics that transforms abstract gas behavior into visual revelation. By plotting pressure against volume at constant temperature, Boyle’s Law Graphs expose a precise inverse relationship: as volume shrinks, pressure rises—and vice versa.
This elegant relationship governs phenomena from balloon compression to scuba diving, and when scrutinized closely, the graph becomes a narrative of molecular chaos and physical order. At the core of Boyle’s Law lies a simple yet powerful equation: P₁V₁ = P₂V₂ Where P represents pressure, and V volume—the constant product reflects nature’s balance in closed systems. Graphically, this manifests as a hyperbolic curve, concave downward, illustrating how a decrease in volume by even a fraction triggers a proportional surge in pressure.
“The mastery of gas behavior begins not with formulas, but with visualizing this inverse dance,” notes Dr. Elena Torres, a senior physicist specializing in thermodynamics. “The Boyle’s Law Graph turns invisible forces into understandable motion.”
Visualizing the Curve: The Shape and Science Behind the Plot
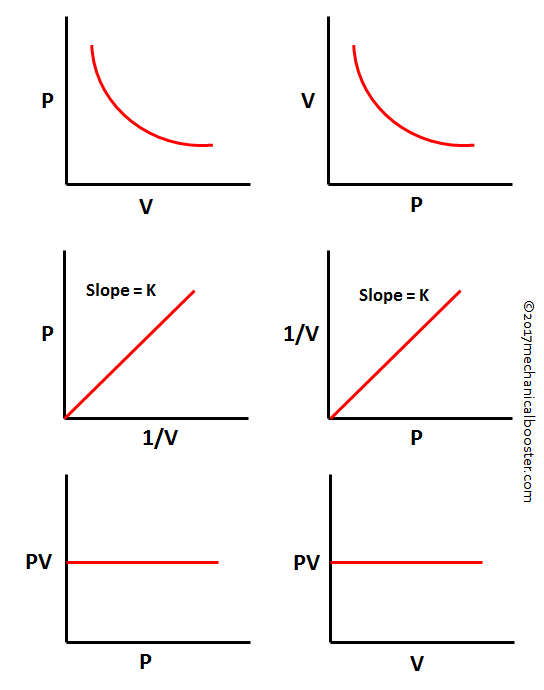
A typical Boyle’s Law Graph plots volume (V) on the horizontal axis and pressure (P) on the vertical, with a smooth, decreasing curve beginning at a wide, low-pressure point.As volume is reduced, pressure skyrockets—imagine the air compressed into a smaller space, with molecules crashing together more frequently, increasing force per unit area. Conversely, expanding volume dilutes pressure, a principle evident in elevator systems, bicycle tires, and even the human respiratory cycle. The curve’s characteristics reveal critical insights: - The x-intercept (volume = 0) lies at infinite pressure—a theoretical boundary showing what happens if space contracts to zero.
- The y-intercept (pressure = 0) occurs at infinite volume, indicating pressure vanishes when molecules have no containment. - The curve’s nonlinearity underscores the nonlinear sensitivity of gas pressure to volume changes—tiny reductions in volume lead to dramatic pressure spikes. “This inverse relationship isn’t just mathematical—it’s a window into molecular dynamics,” explains Dr.
Ramirez, a mechanical engineering professor. “As volume collapses, molecules occupy less space, increasing their collision frequency, which directly amplifies pressure. The graph captures that energetic transfer with stunning clarity.”
Real-World Applications: From Science Labs to Deep Ocean Dives
Boyle’s Law Graph is not merely a theoretical construct—it underpins countless practical technologies and natural processes.In respiratory physiology, the graph explains how the lungs inflate and deflate: during inhalation, thoracic volume expands, reducing internal pressure relative to the outside air, drawing oxygen in. Exhalation compresses the volume, increasing pressure to expel carbon dioxide. “It’s nature’s textbook,” says pulmonologist Dr.
Marcus Liu. “The Boyle’s Law Graph maps exactly what happens in every breath we take.” Outside biology, in scuba diving, the graph governs safe decompression practices. Compressed air tanks hold high-pressure gas; as divers descend and surface, expanding volume in cylinders reduces pressure, but violating safe ascent rates can cause dangerous gas bubble formation—illustrating the law’s critical role in human safety.
Pressure vessels, industrial compressors, and HVAC systems also rely on precise Boyle’s Law calculations to maintain operational integrity.
Mathematical Insights and Graphical Precision
The Boyle’s Law Graph’s shape stems from ideal gas behavior under constrained temperature, formalized during the 17th century experiments of Robert Boyle, whose tensioned air pump laid groundwork for modern thermodynamics. The inverse proportionality means that doubling volume halves pressure—assuming constant temperature.Graphically, this forms a hyperbola: on a linear scale, the curve appears curved but remains strictly decreasing and asymptotic near zero pressure as volume grows. For engineers and educators, the graph serves as an indispensable teaching tool. It bridges numerical data and spatial intuition, allowing students and professionals alike to anticipate pressure shifts without complex calculations.
“When plotted accurately, the graph forecasts pressure changes with surprising immediacy,” states Dr. Torres. “It’s analytical simplicity married with explanatory power.”
Advanced Considerations and Limitations
While Boyle’s Law is precise for ideal gases under isothermal conditions, real-world applications demand nuance.At extreme pressures or temperatures, gases deviate from ideal behavior, and molecular interactions become significant. Quantum and statistical mechanics extend the model to molecular scales, but the Boyle graph remains a robust approximation in appropriate contexts. Moreover, the graph assumes closed systems—no gas exchange with surroundings—and neglected intermolecular forces.
In dynamic systems, heat transfer, non-isothermal shifts, or phase changes introduce complexity. Nonetheless, within its scope, the Boyle’s Law Graph endures as a cornerstone of physical science education and industrial design.
Visual Literacy: Reading Pressure-Volume Relationships
Understanding the Boyle’s Law Graph equips readers to interpret pressure-volume dynamics in both natural and engineered systems.The curve itself becomes a decoder: identifying where volume and pressure trade off illuminates system behavior. Whether troubleshooting a mechanical seal, analyzing respiratory mechanics, or designing safety protocols, professionals rely on this graph to predict and optimize outcomes. “This isn’t just about numbers on a line,” emphasizes Dr.
Ramirez. “It’s about seeing the invisible—how space and force intertwine. When you master this graph, you master a fundamental language of physics.”
The Heart of Gas Behavior: Why Boyle’s Law Graph Endures
Boyle’s Law Graph transcends its mathematical roots to become a living illustration of physical laws in action.It captures the delicate balance of molecular motion and pressure, revealing how small changes in volume rewrite the pressure landscape. From breathing to engineering, its predictive power shapes innovation and safety. More than a curve on a chart, it is a gateway to understanding the silent forces that lift balloons, sustain life, and power industry.
In every shift along the graph, science meets daily experience—underscoring that some of the most profound laws of nature are written not in complex equations, but in the simple, elegant interplay of pressure and volume.


Related Post

Who Invented Television: The Untold Story Behind a Revolutionary Breakthrough

Decode Every Angle: Types of Triangles and Their Defining Properties

Hambuglar: Revolutionizing Sustainable Performance in Modern Markets

