Unlocking the Chemistry of Sulfur Dioxide: The Lewis Structure That Defines Its Reactivity
Unlocking the Chemistry of Sulfur Dioxide: The Lewis Structure That Defines Its Reactivity
Sulfur dioxide (SO₂) stands as one of chemistry’s most industrially significant and structurally intriguing compounds, famously recognized not only for its pervasive presence in volcanic emissions and air pollution but also for its nuanced molecular architecture. Central to understanding its chemical behavior is the Lewis structure—a foundational tool that reveals how sulfur and oxygen atoms share electrons, forming bonds that define SO₂’s unique reactivity and physical properties. More than a mere diagram, the sulfur dioxide Lewis structure exposes the molecular logic behind its polarity, resonance, and environmental impact, making it a cornerstone in both academic study and industrial application.
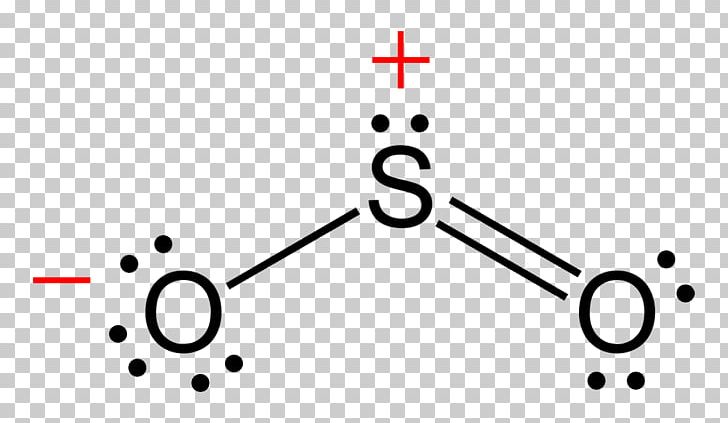
At its core, the Lewis structure of sulfur dioxide illustrates a trigonal planar electron geometry around sulfur, with a bent molecular shape resulting from the presence of one lone pair and two bonding pairs. Sulfur, the central atom, forms double bonds with each of the two oxygen atoms—elaborated by valence electrons distributed across sp² hybrid orbitals. Each S–O bond is a hybrid of a sigma and a pi interaction, giving the molecule partial double-bond character and significant stability.
This configuration directly influences its reactivity: sulfur’s expanded valence shell and willingness to accommodate an extra electron pair explain SO₂’s role as a dynamic oxidizing agent and its ability to readily engage in precursor reactions across the chemical industry.
Electron Distribution and Formal Charges: The Mechanical Precision of SO₂’s Lewis Structure
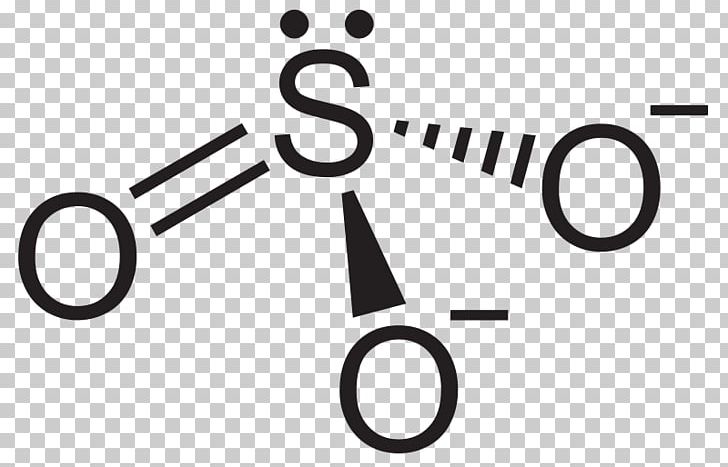
The formal structure of sulfur dioxide is elegantly governed by the principles of valence electron allocation. With sulfur possessing six valence electrons and each oxygen contributing six, the total available electrons amount to 24. In the Lewis model, sulfur forms two double bonds—occupying four electrons—and retains one lone pair, accounting for all 24 electrons without exceeding the octet rule, which sulfur effectively bypasses due to its intermediate position in the periodic table.Oxygen atoms, each completing their octet via two bonds, assume three lone pairs, contributing to the molecule’s overall polarity.
Importantly, formal charge analysis strengthens the Lewis structure’s predictive power. The sulfur atom bears a formal charge of zero, consistent with its stable electron distribution, while each oxygen carries a -1 formal charge, arising from seven valence electrons paired into three lone pairs versus forming two bonds.
This charge localization—negative on oxygen, neutral on sulfur—explains SO₂’s polar nature and its tendency to attract electrophiles such as peroxides and nitric oxide. “The polarity is not just structural—it’s functional,” notes Dr. Elena Vasiliev, inorganic chemist at the Institute of Environmental Chemistry.
“The uneven electron sharing drives SO₂’s reactivity in atmospheric oxidation pathways and its utility in manufacturing sulfur-based intermediates.”
The Lewis structure further reveals a key feature: resonance. Though often depicted as a single representation, SO₂ actually exists in two resonant forms—where the double bond character alternates between one S–O and the other—despite neither configuration overwhelming the other. This delocalization stabilizes the molecule and reduces energy, preventing localized charge buildup.
“Resonance in SO₂ is subtle but vital,” explains Dr. Marcus Lin, a structural chemist at the Global Institute of Inorganic Chemistry. “It lowers the energy barrier for reactions, enabling SO₂ to participate efficiently in industrial oxidation catalysis and environmental chemical cycles.”
Beyond stability, the bent molecular geometry—approximately 119 degrees—emphasizes the impact of lone-pair repulsion.
Formula-driven bond angle calculations confirm that the presence of one lone pair compresses the S–O–S angle relative to a perfect trigonal planar 120°. This angular distortion contributes to the molecule’s dipole moment, intensifying its polarity and enabling strong intermolecular interactions. Such physical consequences translate into measurable impacts: SO₂’s high solubility in water (owing to hydrogen bonding potential via lone pairs) underpins its role in acid rain formation, where uptake into atmospheric moisture drives the creation of sulfuric acid patches that damage ecosystems.
The Lewis model also serves as a springboard for understanding SO₂’s reactivity in synthesis.
From the production of sulfuric acid via contact process to its role as a precursor in fine chemical manufacturing, the electron-sharing dynamics revealed by the structure guide reaction pathways. For instance, the electrophilic nature of sulfur encourages addition to double bonds and participation in nucleophilic substitutions, enabling transformations into sulfites, sulfates, and other sulfur derivatives. “The elegance of the sulfur dioxide Lewis structure lies in how it decodes reactivity,” states Dr.
Vasiliev. “It’s not just about drawing lines—it’s about predicting behavior, designing processes, and safeguarding the environment through better chemical control.”
Environmental concerns underscore the urgency of grasping SO₂’s chemistry. As a primary pollutant, emissions stem largely from fossil fuel combustion, contributing to smog, respiratory hazards, and acid deposition.
The Lewis structure, by illustrating how SO₂ oxidizes in the atmosphere to form SO₃ and then H₂SO₄, provides insight into mitigation strategies—from catalytic converters that reduce SO₂ release to scrubbers that capture emissions at industrial stacks. Understanding molecular behavior at the Lewis level directly informs cleaner, more efficient technologies that reduce ecological harm.
In industrial laboratories, accurate Lewis depiction of SO₂ is foundational for safety and process optimization.
Safety data sheets reference structural properties rooted in electron distribution to assess reactivity risks, while chemical engineers rely on bond polarity and geometry to design reactors, determine solubility limits, and optimize separation techniques. The Lewis structure is thus far more than a static image—it’s a dynamic blueprint actively shaping real-world applications across energy, manufacturing, and environmental sectors.
Ultimately, the sulfur dioxide Lewis structure stands as a paradigm of molecular insight—bridging abstract electron principles with tangible chemical consequences.
It reveals why SO₂ is both resilient and reactive, a molecule oscillating between stability and transformation. Whether in the crucible of industrial synthesis or the atmosphere where pollution meets air and water, this structure endures as a vital tool for scientists, engineers, and policy-makers alike—proving that even the smallest details of molecular architecture hold extraordinary power.

Related Post

What Is The Time of Texas? Unpacking the State’s Complex Time Zone Reality

Prayer Time Eid Ul Adha: The Sacred Moment When Faith Meets Tradition
Iben Shelton: Unveiling His Story On Spanish Wikipedia
Examining the Altitude of The Icon Carter: How Tall Is Jay Z?

