Unlocking the Chemistry of Life: Master Sulfur’s Role Through S Lewis Dot Structures
Unlocking the Chemistry of Life: Master Sulfur’s Role Through S Lewis Dot Structures
Sulfur’s chemistry reveals a quiet yet pivotal role in biology, primarily illuminated through the precise geometry of its Lewis dot structures. These visual representations decode how sulfur atoms bond—offering critical insights into protein synthesis, enzyme function, and metabolic pathways. Unlike many elements, sulfur’s ability to form stable covalent bonds and variable oxidation states hinges on its unique electron arrangement, visually captured in its dot structure.
Understanding these patterns transforms abstract bonding into tangible molecular behavior, making it indispensable for researchers, educators, and students alike.
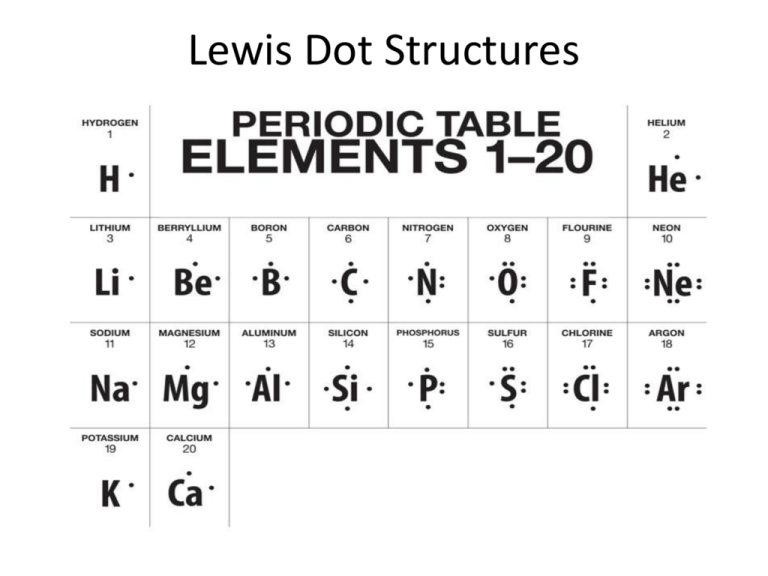
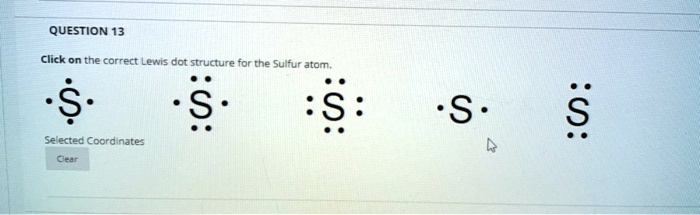
Central to sulfur’s versatility is its electronic configuration: with an atomic number of 16, sulfur possesses six valence electrons arranged as 2s² 2p⁴. These six electrons form three lone pairs and one or two shared pairs when bonding, defining how sulfur integrates into organic and biological systems.
The Lewis structure captures this arrangement, revealing not just simple connections but also sulfur’s capacity to adopt multiple oxidation states—ranging from –2 in sulfides to +6 in sulfates—alongside resonance in compounds like sulfur dioxide. This selectivity in electron sharing underpins sulfur’s role in vital molecules such as amino acids and vitamins.
The Building Blocks: Sulfur in Amino Acids and Proteins
Sulfur is strategically positioned in key biological amino acids—cysteine, methionine, and homsteine—where its Lewis structure dictates both reactivity and structure.Cysteine, for instance, features two sulfur atoms in its side chain, enabling disulfide bridge formation critical to protein tertiary structure. The resonance-stabilized Lewis dot structure of the cysteine side chain allows dynamic electron delocalization, enhancing protein stability through covalent cross-linking. Meanwhile, methionine’s sulfur-bearing methylthio group serves as a methyl donor in metabolic reactions, with its valence electrons enabling predictable nucleophilic behavior.
`sulfur’s role extends beyond static bonds: its Lewis structure reveals adaptability. In cysteine, equivalent sulfur atoms permute in disulfide bonds due to geometric flexibility, stabilizing enzymes and cell membranes. This dynamic bonding, visualized through electron distribution, underscores sulfur’s centrality in structural and functional biology.
Resonance, Oxidation States, and Reactivity
The sulfate ion (SO₄²⁻) exemplifies sulfur’s diverse bonding through multiple resonance structures, each illustrating equal bond length and electron delocalization.In this polyatomic ion, sulfur shares electrons across four oxygen atoms, with oxidation state –6 reflecting full electron contribution. Unlike static depictions, modern Lewis models highlight resonance hybridization—a higher-energy, exchangeable arrangement—explaining sulfate’s role in electron transfer and energy metabolism.
Sulfur’s oxidation states—ranging from –II in hydrogen sulfide (H₂S) to +VI in sulfuric acid—mirror dramatic shifts in electron distribution.
The Lewis structure of H₂S, with two single S–H bonds and lone pairs, shows sulfur in a reduced state, donating electrons with relative ease. In contrast, the +6 state in SO₄²⁻ depicts maximal electron withdrawal, stabilized by oxygen’s high electronegativity. These transitions, visible in bond angles and formal charges, explain sulfur’s function in redox reactions, from biochemical catalysis to industrial oxidation processes.
Applications Beyond the Lab
Sulfur’s bonding insights extend into pharmacology, agriculture, and environmental science. In drug design, sulfur’s Lewis structure guides rational synthesis—enabling inhibitors that target disulfide bond formation in disease-related proteins. In agriculture, understanding sulfur’s oxidation state chemistry aids fertilizer development, ensuring efficient plant uptake.Environmentally, sulfur cycling—from atmospheric SO₂ to sulfates in soil—relies on redox dynamics explained by electronic structure. Each application hinges on the fundamental clarity of Lee Nash structures, transforming abstract bonding into tangible utility.
From the atomic scale to industrial processes, S Lewis Dot Structures offer a timeless lens through which to understand sulfur’s chemistry.
They move beyond symbolism to reveal how electron arrangement governs real-world reactivity, stability, and function. In an era of precision science, these models remain foundational—bridging theory and application with unmatched precision.
Final Thoughts: Why Sulfur Matters—Expressed in Structure
Through the precise geometry of S Lewis Dot Structures, sulfur emerges not as a background element, but as a dynamic player in biochemistry and materials science. Its ability to form stable, versatile bonds—validated by electron distribution, oxidation states, and resonance—fuels life’s complexity and technological innovation alike.Mastery of sulfur’s bonding patterns empowers scientists to predict, manipulate, and harness its potential, proving that even the smallest atoms shape the largest scientific narratives.


Related Post
Victory Brinker Singer Bio Wiki Age Family Siblings Adopted Disability AGT and Net Worth

Sunday Night Football Time Schedule and How to Watch It—Everything You Need to Know in 2024

What Is There To Do In Jackson Hole? A Year-Round Paradise of Adventure, Culture, and Wildlife
CM Punk Compares Becky Lynch Asuka To Batman Joker

