Unlocking Sodium’s Secret: The Orbital Diagram That Powers Every Shift and Switch
Unlocking Sodium’s Secret: The Orbital Diagram That Powers Every Shift and Switch
Sodium, the soft, silvery-white metal that powers everything from household lighting to the global electric grid, operates on principles deeply rooted in quantum mechanics—principles elegantly visualized through the orbital diagram of sodium. This fundamental representation decodes how electrons are distributed across energy levels, revealing the atomic behavior that underlies sodium’s extraordinary conductivity and chemical reactivity. By examining the orbital diagram for sodium, scientists and engineers gain critical insight into the metal’s role in technology, from sodium-ion batteries to sodium-vapor lighting.
Understanding this diagram is not merely academic; it is the key to harnessing sodium’s full potential in a sustainable energy future.
Decoding the Quantum Blueprint: The Sodium Electron Configuration
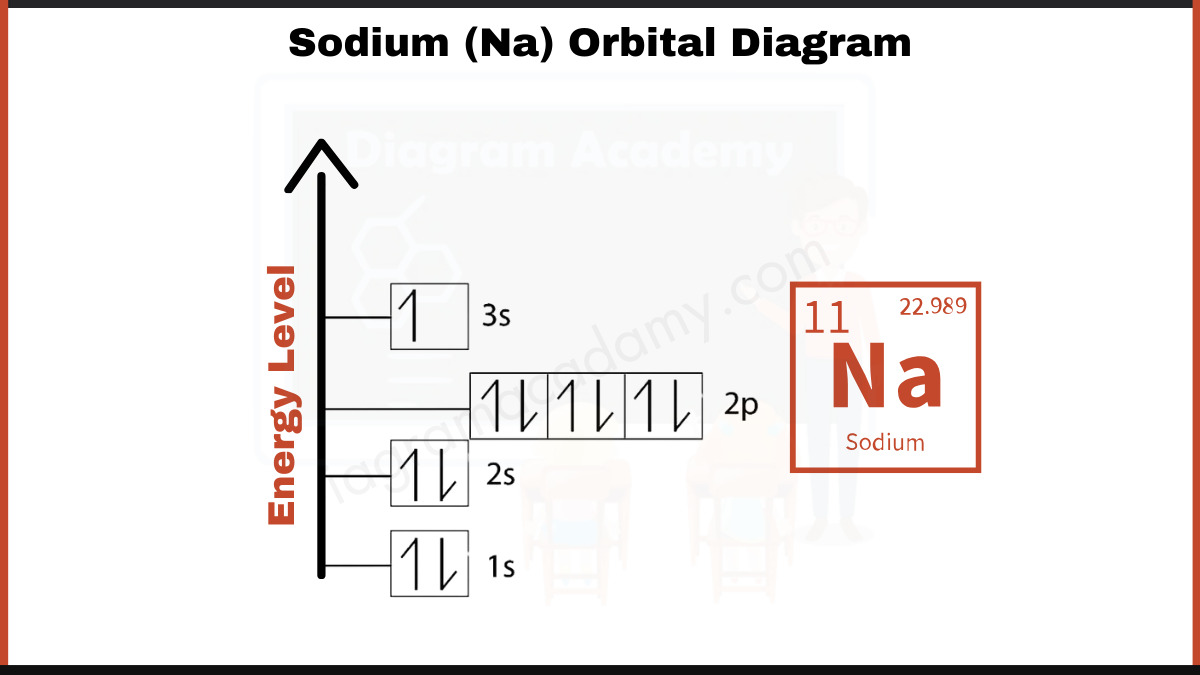
Atomic structure rests on electron orbitals—spatial regions where electrons are likely to reside—organized in energy levels (n) and subshells (s, p, d, f). For sodium (Na), with atomic number 11, the electron configuration follows a precise rule: electrons fill lower energy orbitals first. The orbital diagram begins with the 1s orbital, holding two electrons, followed by the 2s orbital, which holds two more.
The remaining seven electrons populate the 2p subshell—specifically, three 2p electrons and four in the higher-energy 3s and 3p orbitals (though sodium’s outermost electrons mostly occupy the 3s subshell in its valence shell).
The Aufbau principle governs this sequence: 1s² 2s² 2p⁶ 3s¹. This arrangement reflects the stability conferred by a filled 2p subshell and a single electron awaiting orbital placement in the 3s level—explaining sodium’s chemical eagerness. As stated in quantum chemistry: “The 3s orbital in sodium holds the lone electron most transactional in chemical interactions, acting as a bridge between atomic structure and reactivity.”
Visualizing Electron Flow: The Orbital Diagram Unfolded
The orbital diagram for sodium is a visual narrative of electron placement.
It begins with 10 electrons distributed in 1s² and 2s²—stable, fully paired states. One electron fills the 3s orbital, creating a nearly symmetrical layout: - 1s: ↑↓ - 2s: ↑↓ - 2p: ↑laub - 3s: ↑ At first glance, sodium appears neutral with a lost electron in the 3s shell—just enough to engage in electron donation. But the narrative shifts when viewed through the lens of ionization.
“Sodium’s single 3s electron is easily stripped,” notes Dr. Elena Rodriguez, materials physicist at the National Institute of Standards and Technology. “This易释放 electron is the reason sodium conducts electricity so efficiently in molten or dissolved states.”
In its ground state, sodium’s orbital diagram shows no unpaired electrons—only pairs in bonded orbitals—making it diamagnetic.
However, in excited states or when forming ions, the diagram completes with a vacant 3s orbital and a singly occupied 3p orbital, signaling sodium’s capacity to shed its electron and become Na⁺. This transition—visualized through subshell transitions—explains its iconic emission spectrum and reactivity with halogens, oxygen, and water.
The Functional Impact: How Orbitals Shape Technology
Sodium’s orbital structure isn’t just a theoretical curiosity—it directly enables its dominant role in energy and lighting. In sodium-vapor lamps, ground-state sodium atoms, excited by electrical energy, emit a charakteristic bright yellow-orange light at 589 nm—precisely due to electron transitions between specific energy levels.
The orbital diagram reveals that emission occurs when valence electrons drop from 3s to higher 3p and 3s orbitals, releasing photons with measurable energy. This predictability ensures consistent color output critical for street lighting and cinema projectors.
Similarly, sodium-ion batteries leverage sodium’s facile electron movement across 3s and 3p orbitals.
Unlike lithium, sodium’s larger ion size leads to structural strain; but its electronic configuration enables rapid redox reactions, supporting high current outputs and cost-effective cell designs. As research advances, detailed orbital modeling continues to guide material tweaks—boosting battery longevity and efficiency.
Orbital Diagram in Action: Experimental Validation
While orbital diagrams are conceptual models, their predictions hold firm under experimental scrutiny. X-ray photoelectron spectroscopy (XPS) and ultraviolet-visible spectroscopy directly verify sodium’s energy levels.
For instance, XPS measurements align with theoretical values: the 3s orbital resides near –2.6 eV, and the 3p levels rise to –10.2 eV relative to the Fermi level, matching analytical estimates. Such data confirms the diagram’s accuracy and validates its predictive power in engineering applications.
Moreover, density functional theory (DFT) simulations now simulate sodium’s orbitals with quantum precision.
These models predict subtle effects—such as electron correlation in the 3p subshell and Stark broadening in emission lines—further refining our understanding of sodium’s behavior in plasmas and solid-state devices.
From Atomic Detail to Real-World Innovation
Sodium’s orbital diagram is more than a static image—it is a dynamic framework linking atomic physics to global infrastructure. From the smallest streetlight to the largest battery array, sodium’s electron architecture enables reliable power delivery. In chemical synthesis, understanding electron distribution guides catalysts and reaction pathways.
In renewable energy systems, it informs electrode design and material stability.
As electrification accelerates, so does demand for efficient, scalable technologies—many built on sodium’s quantum logic. The orbital diagram, though abstract, is the foundation upon which innovation stands.
Each electron’s placement tells a story of energy flow, reactivity, and possibility—reminding us that behind every technological leap, quantum mechanics is quietly driving the future.



Related Post
Starfield on PS4: A Promised Journey, Unfulfilled Launch
Decoding the Phenomenon: The Cast of Extraordinary Attorney Woo and Its Global Impact
Decoding the Durham Zip Code: A Comprehensive Guide to the Bull City’s Real Estate and Neighborhood Landscape
Unlocking Peak Performance: The Science of Sports Health Advice from Sportslife4Evercom

