Unlocking Molecular Complexity: The Science and Significance of Trigonal Pyramidal Compounds
Unlocking Molecular Complexity: The Science and Significance of Trigonal Pyramidal Compounds
At the heart of organic chemistry and pharmaceutical innovation lie trigonal pyramidal compounds—distinct structural motifs defined by a central atom bonded to three ligands and carrying a lone pair of electrons, adopting a tetrahedral electron geometry and a characteristic pyramidal molecular shape. These compounds, best exemplified by ammonia (NH₃) and potassium hydride (KH), play pivotal roles in chemical reactivity, catalysis, and drug design, offering profound insights into molecular behavior and function. Their unique geometry, driven by VSEPR theory, underpins critical intermolecular interactions and stereochemical control, making them indispensable in both fundamental research and industrial applications.
The Geometry and Bonding Behind Trigonal Pyramidal Structures
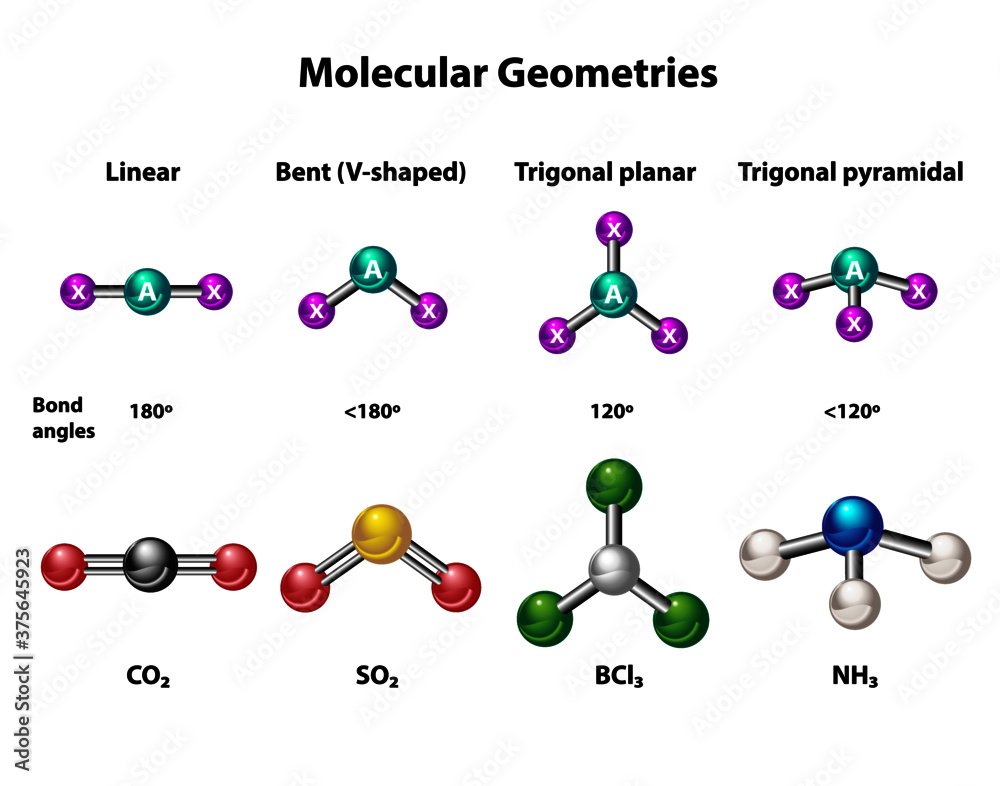
In trigonal pyramidal compounds, the central atom occupies one vertex of a tetrahedron formed by its three bonded atoms and one lone pair of electrons.
This lone pair, oriented in a direction that minimizes electron repulsion, pushes the bonded atoms slightly closer together, creating a positive charge density at the apex and a net electron density at the molecular center. The VSEPR principle predicts this 109.5° bond angle, slightly compressed from the ideal tetrahedral 109.5° due to lone pair–bond pair repulsion—often described as “electron crowding” at the core. This structural configuration is a sterically constrained yet dynamically active arrangement, enabling these molecules to act as nucleophiles, bases, and ligands in diverse chemical environments.
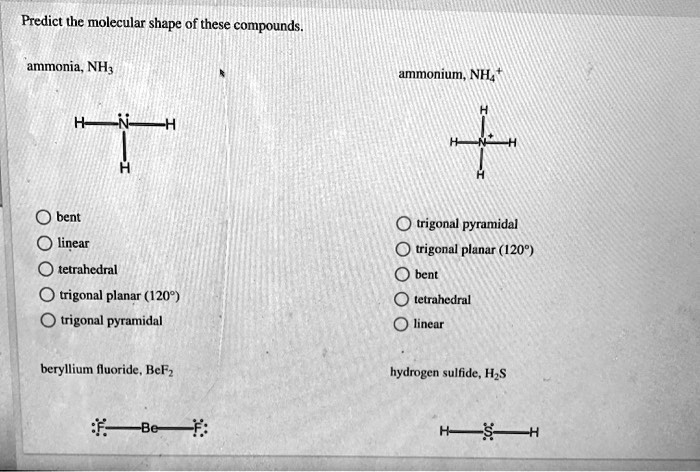
Consider ammonia (NH₃), a prototypical trigonal pyramidal molecule. With a nitrogen atom bonded to three hydrogen atoms and possessing one lone pair, NH₃ exemplifies how molecular geometry influences reactivity. The lone pair enhances nitrogen’s nucleophilicity, making ammonia a key reagent in synthesis such as the production of ammonium salts and amines.
Similarly, in organometallic chemistry, methylammonium ions derived from such compounds serve as chiral precursors in asymmetric catalysis, where precise three-dimensional control is essential.
Key Compositions and Industrial Applications
Trigonal pyramidal compounds span a broad range of chemical classes, each with unique properties and applications. Ammonium derivatives, including ammonium carbonate and ammonium phosphate, are foundational in fertilizers, leveraging their stability and solubility to enhance nutrient uptake in agriculture. Potassium hydride (KH), another well-known example, functions as a strong base and reducing agent in organic synthesis, particularly in Grignard reactions and enolate formations.
pioneers in pharmaceutical chemistry exploit these structural motifs in drug development. The lone pair on nitrogen in compounds like certain serotonin receptor agonists enables selective binding through hydrogen bonding, enhancing drug-target specificity. Additionally, trigonal pyramidal ligands coordinate with transition metals in catalytic systems, improving efficiency in hydrogenation and cross-coupling reactions.
For instance, N-heterocyclic carbenes (NHCs), though often planar, can adopt pyramidal conformations under strain, facilitating robust metal-ligand bonds in industrial catalysis.
In materials science, these compounds contribute to the design of functional polymers and ionic liquids. Ammonium-based ionic liquids, derived from trigonal pyric structures, exhibit low volatility and high thermal stability, making them ideal for green solvents and electrochemical applications such as batteries and fuel cells.
Their tunable properties—adjustable by modifying substituents on the central atom or ligands—allow engineers to craft materials with precise physical and chemical behavior.
Safety, Handling, and Environmental Considerations
Despite their utility, trigonal pyramidal compounds often pose handling and environmental challenges. Ammonia, for example, is highly volatile and toxic at low concentrations, requiring stringent safety protocols in industrial settings. Proper ventilation, personal protective equipment, and secondary containment systems are essential to prevent inhalation risks and accidental spills.
From an environmental standpoint, nitrogen-containing pyramidal compounds like ammonium can contribute to eutrophication if released untreated into water systems, spurring development of advanced wastewater treatment technologies. Innovations in catalytic nitrate reduction and bioremediation leverage microbial pathways to convert ammonium into harmless nitrogen gas, aligning industrial practices with sustainability goals. Regulatory frameworks, including those by the Environmental Protection Agency (EPA), emphasize closed-loop recycling and minimal discharge to protect ecosystems.
Current Research Frontiers and Future Directions
Ongoing research into trigonal pyramidal compounds is uncovering novel reactivities and applications.
Computational chemistry and advanced spectroscopy—such as X-ray crystallography and NMR—reveal subtle ligand effects and non-covalent interactions previously inaccessible. Scientists are designing chiral pyramidal ligands for enantioselective catalysis, aiming to produce pharmaceuticals with greater precision and less waste.
In metaphysics of molecular design, researchers are exploring hybrid structures that combine trigonal pyramidal motifs with other dipolar systems, such as bent or fulvarent geometries, to create responsive materials capable of withstanding dynamic environments.
These developments could revolutionize adaptive polymers, stimuli-responsive drug delivery vehicles, and smart coatings with self-healing properties.
As quantum computing matures, theoretical models are simulating electron density distributions in these compounds with unprecedented accuracy, potentially revealing new reaction pathways and metastable states. Such breakthroughs promise to accelerate discovery while minimizing experimental trial and error, optimizing resource use in chemical innovation.
Trigonal pyram

Related Post
HBCUs in North Carolina: Pivotal Institutions Shaping Education and Community in the Tar Heel State

Build Amazing Minecraft Survival Homes: The Ultimate Beginner’s Guide to Thriving in the Block Universe

Winden Business Debit Settlement: Master Business Transactions with Confidence
Convert Dollar to RandExchange Rate

