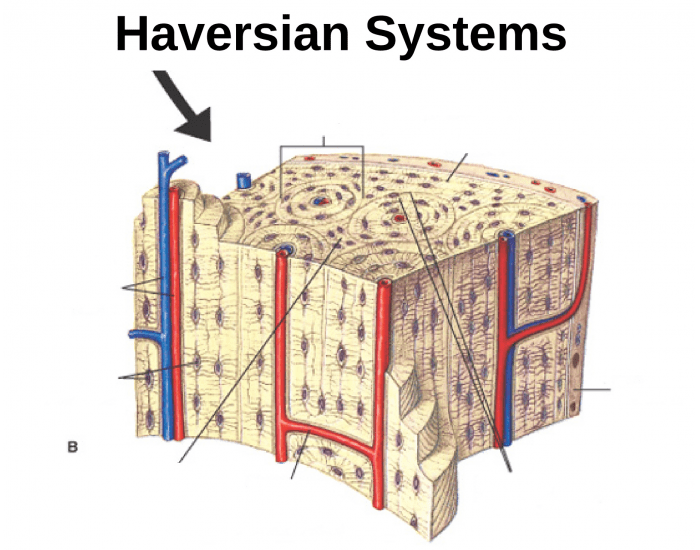
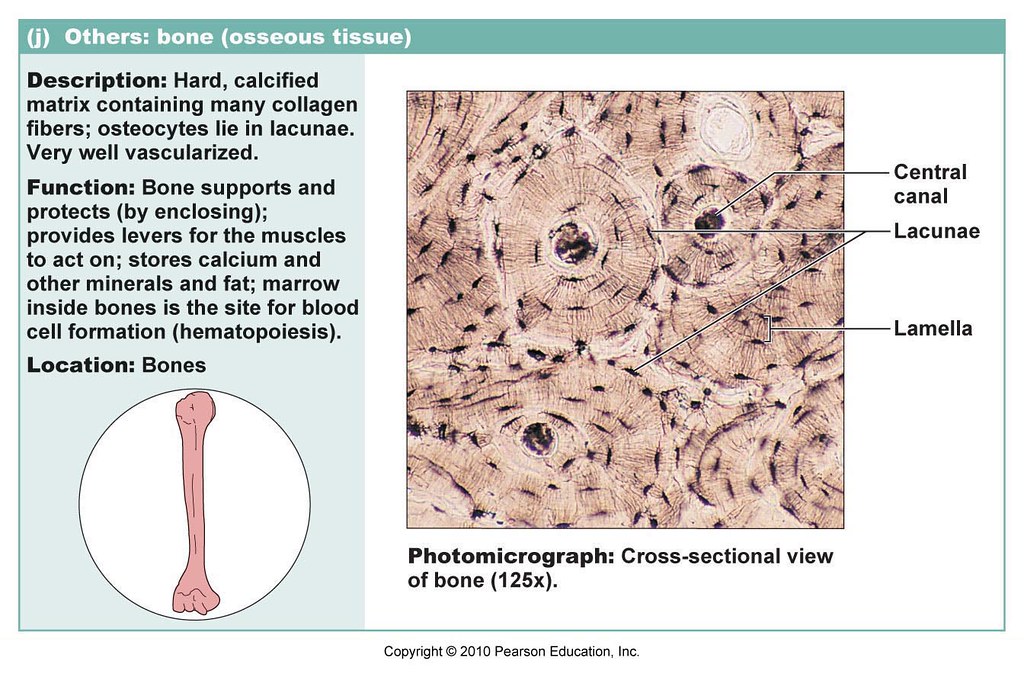
Unlocking Medical Secrets: The Vital Role of Central Canal Bone Tissue in Cranial Health
Unlocking Medical Secrets: The Vital Role of Central Canal Bone Tissue in Cranial Health
Beneath the complex architecture of the human brain lies a network of microscopic wonders, among which the central canal bone tissue plays a surprisingly pivotal role in maintaining central nervous system integrity. This often-overlooked tissue, embedded within the neurocranium, contributes fundamentally to both structural stability and dynamic physiological function. Far more than a passive structural element, central canal bone tissue supports neural health through its unique composition, mechanical properties, and integration within the brain’s protective framework.
Spanning key regions from the ventricular system to deeper cerebral parenchyma, central canal bone tissue resides primarily within the periventricular zone where the cerebral aqueduct gives way to the ependymal lining. It consists of a specialized assemblage of mineralized and non-mineralized matrices—primarily collagen type I, hydroxyapatite crystals, and specialized ependymal cells—that collectively form a biomechanically active, physiologically responsive barrier. Unlike conventional cortical bone, this tissue maintains a finely tuned porosity, allowing for controlled exchange of cerebrospinal fluid (CSF) and supporting the blood-CNS interface.
This structural duality—rigid yet dynamic—enables it to buffer mechanical stress while facilitating vital fluid circulation.
The Dynamic Interface: Central Canal Bone and Cerebrospinal Fluid Exchange
Central canal bone tissue is not merely a structural scaffold; it actively regulates CSF dynamics crucial to brain homeostasis. The ependymal cells lining the central canal exhibit coordinated ciliary movement that propels CSF along the ventricular system, a process influenced directly by the permeability and mineral density of the surrounding bone matrix. Research indicates that subtle variations in the mineralization patterns of this tissue can alter local fluid resistance, affecting both CSF flow velocity and solute transport efficiency.Key findings from current neurooccupancy studies:
- The central canal’s mineralized perivascular septa exhibit a honeycomb-like porous network with average pore sizes measuring 5–15 micrometers, sufficient for plasmalemmal vesicle transport but restrictive for large protein aggregates.
- This selective permeability helps maintain a low-protein milieu in close proximity to brain parenchyma, reducing neuroinflammatory triggers.
- A thin layer of mineralized ependymal membrane at canal edges reinforces containment while enabling nutrient diffusion.
“The central canal’s bone tissue functions as both a mechanical buffer and a dynamic filter—where structural form enables physiological function without sacrificing protective integrity,” says Dr.
Elena Rostova, a neurobiomechanics researcher at the Schwann Institute.
Mineral composition and matrix remodeling are tightly regulated. The central canal bone incorporates osteoinductive factors such as RANKL and osteopontin, which modulate local osteoclast activity to balance resorption with reparative deposition. This homeostatic mechanism prevents pathological calcification while preserving canal patency—essential in aging and neurodegeneration contexts where disrupted mineral balance correlates with CSF flow impairment.
Clinical Significance: Pathologies Linked to Central Canal Bone Dysfunction
Alterations in central canal bone tissue integrity are increasingly implicated in several neurological disorders.For instance, hydrocephalus—characterized by aberrant CSF accumulation—often coincides with abnormal mineralization and reduced permeability in the central canal region. Postmortem analyses reveal ectopic calcifications within the canal lumen in up to 40% of idiopathic normal-pressure hydrocephalus cases, disrupting flow and compressing adjacent neurones.
Similarly, perturbations in bone-tissue dynamics may contribute to the progression of neurodegenerative conditions like multiple sclerosis and Alzheimer’s disease. Elevated levels of sclerotic bone markers in cerebrospinal fluid suggest maladaptive remodeling processes that compromise the canal’s protective capacity.
Moreover, traumatic brain injuries that induce dural erosion sometimes extend microfractures into the central canal bone, triggering inflammatory cascades and scarring that impair CSF resorption.
Early detection via high-resolution MRI "quantitative susceptibility mapping" now enables clinicians to assess subtle changes in bone density and matrix integrity before clinical symptoms emerge.
- Idiopathic normal-pressure hydrocephalus: Characterized by CSF flow obstruction; often linked to disrupted central canal bone matrix porosity and ependymal ciliary dysfunction.
Multiple sclerosis: Bone tissue mineralization anomalies correlate with lesion progression and white matter breakdown.
Post-traumatic cerebrospinal fluid leaks: Fractures extending to canal walls initiate mineral deposition and inflammation, altering local microenvironment.
Emerging Diagnostic and Therapeutic Frontiers
Innovations in neuroimaging and tissue engineering are transforming how central canal bone tissue is studied and treated. Modern diffusion MRI protocols combined with magnetic resonance spectroscopy offer non-invasive visualization of bone matrix mineralization and fluid transport dynamics in vivo. These tools reveal microarchitectural deviations decades before symptom onset, enabling preemptive intervention strategies.On the therapeutic side, biomimetic scaffolds replicating central canal bone’s nanoscale architecture are under development to restore disrupted fluid flow in hydrocephalus patients.
Additionally, targeted modulation of bone-resorbing pathways using RANK pathway inhibitors shows promise in preclinical models for preserving canal patency and CSF homeostasis.
“Understanding the central canal bone tissue is no longer a niche pursuit—it’s a frontier in safeguarding neurological resilience,” explains Dr. Rostova. “Its role transcends mere structure; it is a dynamic participant in brain fluidomics and neuroprotection.”
Harnessing the full potential of central canal bone tissue demands interdisciplinary collaboration—integrating neuroanatomy, biomechanics, molecular biology, and clinical neurology.
As research advances, this tiny but mighty tissue emerges not only as a protector of the brain’s fluid environment but as a promising target for treating some of neurology’s most challenging conditions. Precision diagnostics guided by bone-tissue biomarkers and tailored regenerative therapies stand to redefine care for patients affected by cerebrospinal flow disorders and degenerative brain diseases alike.
In the delicate ecosystem of the central nervous system, central canal bone tissue pulses with quiet significance. Its contribution, though embedded within skull bone, resonates powerfully through the flow of cerebrospinal fluid, the balance of neural health, and the future of neurotherapeutics—proving that sometimes, the most profound medical breakthroughs lie in the smallest, most overlooked structures.

Related Post

Hotmail vs. MSN: Unlocking the Legacy of America’s Pioneering Email Services

From Sticks to Stacks: Mastering Half Stick Butter to Cups
Gratiela Brancusi Roma: A Symphony of Tradition in Romania’s Cultural Landscape

Luxe Approaches to Educational Innovation: Redefining Learning Through Precision, Personalization, and Promise

