Unlocking Light: How Electron Energy Drives the Absorption and Emission of Light
Unlocking Light: How Electron Energy Drives the Absorption and Emission of Light
The dynamic interaction between electron energy levels and light lies at the heart of modern physics and chemistry, powering technologies from lasers to solar cells and explaining the stunning colors of the natural world. By examining the relationship between electron excitations, transitions, and photon behavior through frameworks like the Electron Energy and Light POGIL answer key, students and scientists alike uncover fundamental principles governing how light is absorbed, emitted, and transformed. This exploration reveals the precise mechanisms that govern atomic and molecular light interactions, offering profound insight into both quantum phenomena and real-world applications.
The Electron-Energy Fundamentals and Light Absorption
At the core of light-matter interactions is electron energy—a quantized property determined by atomic structure.
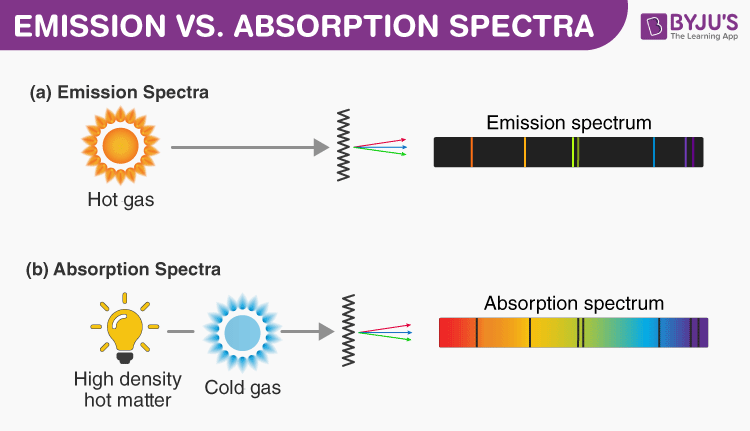
Electrons occupy specific energy levels, with energy differences corresponding to discrete wavelengths of light. According to the POGIL construct, when a photon strikes an atom, its energy must exactly match the energy gap between electron orbitals for absorption to occur. "An electron can only jump to a higher energy level if the photon energy equals the binding energy difference—this is resonance in action," explains a key principle in electron-energymodule.
This selective absorption underpins how elements exhibit unique spectral signatures, enabling applications such as fingerprinting elements in materials science and astronomy.
Absorption spectra serve as blueprints of electron behavior. For example, in a hydrogen atom, electrons transition between principal quantum levels (n = 1, 2, 3...), absorbing photons of specific energies in a predictable pattern. The POGIL answer key emphasizes that absorption peaks correspond directly to the quantized energy differences: ΔE = hν, where h is Planck’s constant and ν is frequency.
When these transitions happen, electrons momentarily occupy higher energy states, storing energy that will later be released as light.
Electron Excitation and Light Emission: From Absorption to Light Emission
Once an electron absorbs energy and reaches an excited state, it is unstable and rapidly returns to a lower energy level—a process that releases energy in the form of emitted light. This emission is governed by the same quantum rules that drive absorption: energy differences between levels dictate the photon’s wavelength, as defined by ΔE = hν.
The Electron Energy and Light POGIL guide highlights this transformation as a fundamental loop in photonic energy exchange.
Emission occurs via two dominant processes: fluorescence and phosphorescence, differentiated by electron spin and transition types. Fluorescence, common in many molecules, involves fast emission from singlet excited states. Phosphorescence, slower and longer-lived, arises from transitions involving metastable triplet states, where quantum spin rules delay release.
Both processes underscore the intimate link between electron energy dynamics and light behavior.
The emitted photon’s wavelength reveals critical information about the system: longer wavelengths indicate lower energy emissions, while shorter ones signal higher energy release. This principle enables precise spectroscopic analysis. As the POGIL framework emphasizes, “The color of light emitted is not random—it’s the DNA of the electron’s journey through excited states,” making light emission a powerful window into quantum rules.
Real-world examples include fluorescent light bulbs, where electrons in mercury vapor absorb UV energy and emit visible light, and in biological systems like chlorophyll, where electron transitions drive photosynthesis and sunlight conversion.
Each emission spectrum acts as a unique signature, decoded by scientists using electron energy knowledge to weigh, measure, and manipulate light interactions.
Examining the Electron Energy and Light POGIL Answer Key: Key Insights and Application
The Electron Energy and Light POGIL answer key serves as a rigorous toolkit that merges conceptual understanding with hands-on analysis. These exercises target core competencies by guiding learners through systematic reasoning about electron excitations, photon interactions, and spectral identification. This structured approach transforms abstract quantum concepts into tangible skills.
Responses emphasize linking electron energy differences directly to observed light behavior.
For instance, learners practice predicting emission wavelengths from given transitions using the formula λ = hc/ΔE, where c is the speed of light. This reinforces the mathematical rigor essential for accurate interpretation of spectroscopy data.
Another core component includes error analysis—students evaluate why non-resonant photon absorption fails, examine spectral line widths influenced by environmental factors, and interpret deviations from ideal models. “Mistakes in energy calculation aren’t failures—they’re discovery opportunities,” the POGIL guide insists, promoting deep engagement over rote memorization.
The key also stresses cross-disciplinary connections: from quantum chemistry to engineering design.
Applications range from developing LED technologies with tailored emission profiles to environmental monitoring via emission spectroscopy of atmospheric pollutants. Each example illuminates how foundational electron-energy principles translate into innovation.
Practical Takeaways from the POGIL Framework
Understanding electron energy and light interplay yields powerful benefits across fields. In material science, tuning electron transitions enables creation of semiconductors and optoelectronic devices.
In chemistry, spectral fingerprints allow precise identification of molecular structures. In renewable energy, photosynthesis-inspired designs leverage electron excitation to convert sunlight efficiently into electricity.
The POGIL framework strengthens this mastery by blending inquiry-based learning with conceptual rigor. Students move beyond definitions to analyze, predict, and synthesize—skills essential for scientific advancement.
“Light and electrons are not just physics concepts—they are the building blocks of technology and life,” notes educators using the POGIL method to bridge theory and real-world impact.
The Future of Electron-Light Interactions: From Quantum to Global
As quantum technologies and sustainable energy solutions evolve, understanding electron energy transitions emerges as a linchpin. Advances in nanophotonics and quantum computing depend on precise control of electron-photon coupling, where the POGIL framework offers foundational clarity. In global challenges such as climate change and clean energy, this knowledge fuels innovations in light harvesting and emission efficiency.
Each lesson, each data point illuminated through electron energy and light interactions, propels deeper insight into nature’s most fundamental forces.
With the POGIL answer key guiding thoughtful inquiry, learners become not just observers but architects of tomorrow’s scientific breakthroughs—turning the invisible dance of electrons and photons into tangible, transformative power.



Related Post

Is Sekai Taikai Real Unveiling the Truth? Inside the Controversial Documentary Exposing China’s Secrecy
Mugshot of Sandra Bland: The Chilling Image That Ignited a Nation’s Demand for Justice

