Unlocking Life’s Tiny Engines: The Critical Role of K₁ Enzyme Kinetics in Biochemical Precision
Unlocking Life’s Tiny Engines: The Critical Role of K₁ Enzyme Kinetics in Biochemical Precision
Enzymes are nature’s molecular architects, driving life’s fundamental reactions with remarkable efficiency and specificity. At the heart of understanding their function lies K₁ enzyme kinetics—the quantitative study of how enzymes bind substrates and transform them into products over time. This field is not just a niche curiosity but a cornerstone of biochemistry, pharmaceutical development, and industrial biocatalysis.
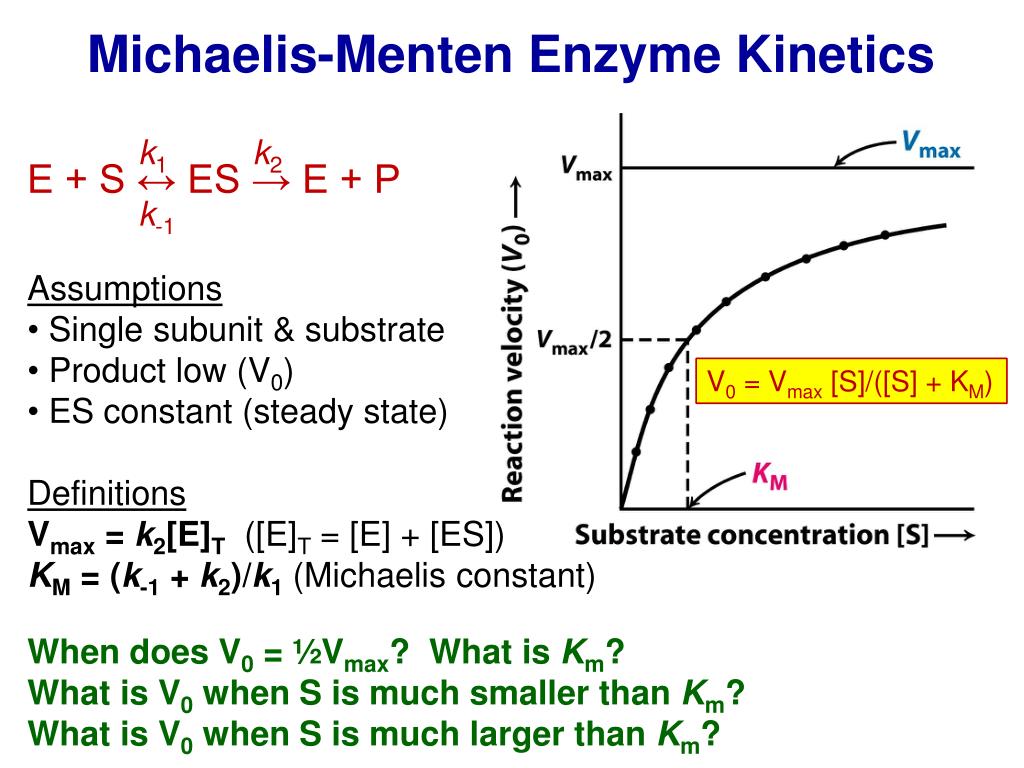
By analyzing the K₁ constant—a measure of enzyme-substrate affinity—researchers decode the speed and binding strength that determine everything from cellular metabolism to drug efficacy. K₁, formally defined as the inverse of the Michaelis constant (Kₘ) under zero-substrate conditions, represents the milestone where half the maximum reaction rate (Vₘₐₓ) is achieved. Though often overshadowed by Kₘ in mainstream discourse, K₁ remains essential for dissecting enzyme behavior at the molecular level.
Its utility rests in its mathematical precision and biological relevance: it quantifies how tightly an enzyme binds its substrate, a factor that influences both reaction velocity and regulation within living systems. As biochemist William Ashley once noted, “The strength of an enzyme’s grip on its substrate dictates the rhythm of life’s chemistry.”
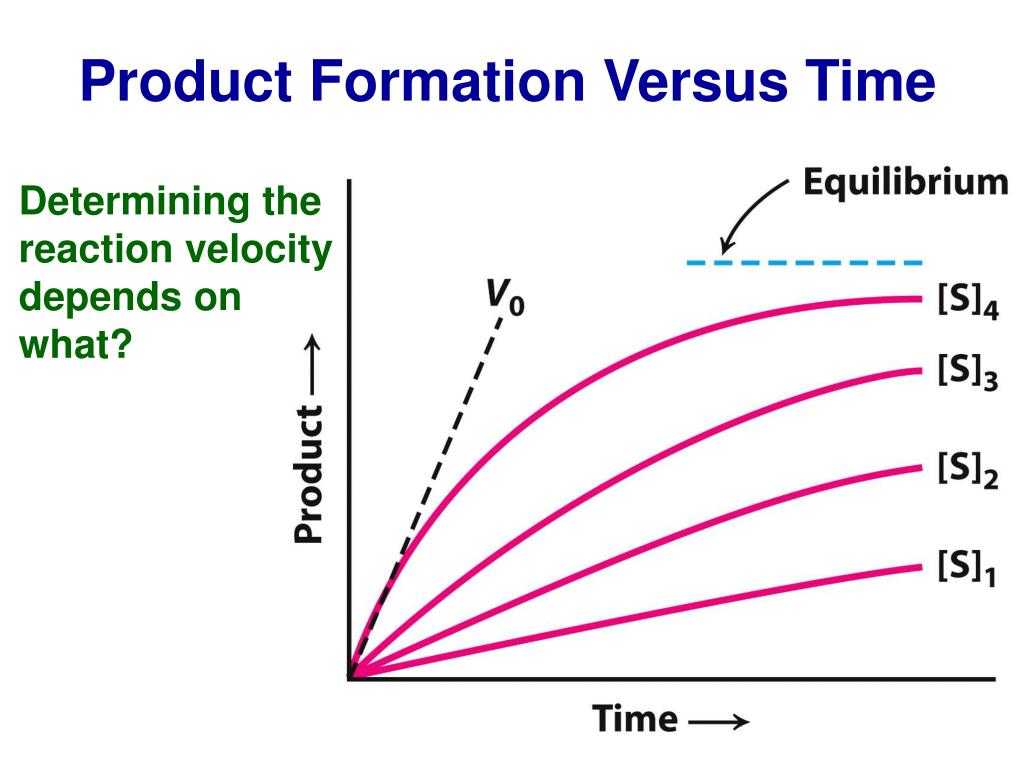
At the core of K₁ enzyme kinetics is the steady-state assumption, which posits that substrate concentration remains constant during the initial phase of catalysis. This simplification allows scientists to model the relationship between substrate concentration and reaction rate with remarkable clarity.
When tracking the formation of product over time, the moment when velocity reaches half its maximum velocity defines the K₁ value. This transition point reflects not only binding affinity but also the energetic barrier enzymes must overcome—a key determinant of catalytic efficiency. Understanding K₁ behavior enables researchers to compare enzymes across species, conditions, or engineered variants.
For instance, in industrial applications, enzymes used in biofuel production or pharmaceutical synthesis must exhibit high K₁ affinity to function effectively under variable substrate concentrations. In clinical settings, K₁ kinetics inform drug design by identifying inhibitors that alter substrate binding—paving the way for targeted therapies.
Breaking Down the K₁ Constant: Definition and Measurement
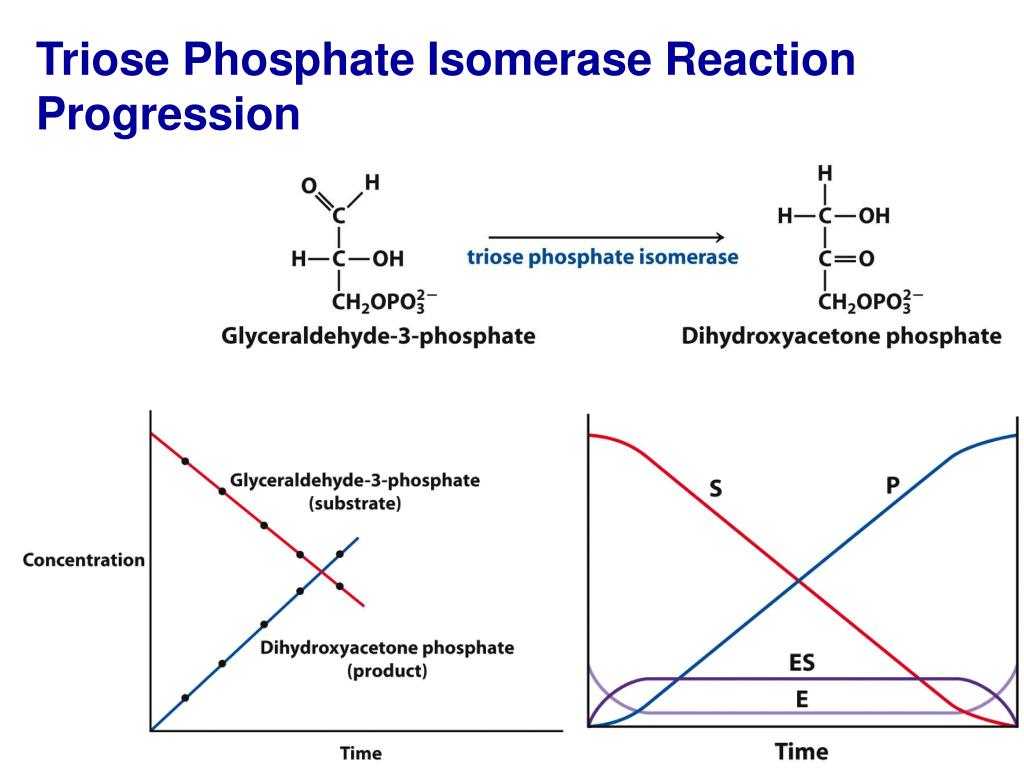
The K₁ constant quantifies the rate at which an enzyme binds its substrate in the absence of free substrate—a microsecond snapshot of binding affinity.Unlike Kₘ, which incorporates competitive inhibition effects, K₁ isolates the intrinsic association rate between enzyme E and substrate S. Mathematically expressed as K₁ = k₋ₑₛ / k₊ₑₛ (where k₋ₑₛ and k₊ₑₛ are forward and reverse binding rate constants), it captures the transient equilibrium before catalysis begins. Measuring K₁ requires precise kinetic experiments, typically performed using rapid reaction techniques such as stopped-flow spectroscopy, steady-state assays, or real-time monitoring with fluorescence or absorbance.
These methods enable detection of minute changes in product formation, translating raw data into kinetic parameters with high reproducibility. A critical advancement in recent years has been the use of surface plasmon resonance (SPR) and single-molecule detection, which offer unprecedented resolution in binding studies—allowing scientists to observe enzyme-substrate interactions with nanosecond precision.
The reliability of K₁ values depends on meticulous experimental control: maintaining constant temperature, pH, and enzyme concentration is paramount.
Small deviations can skew results, particularly in low-substrate regimes where binding becomes rate-limiting. To enhance accuracy, researchers often complement steady-state measurements with pre-steady-state methods that capture the initial burst of product formation, often revealing mechanistic subtleties masked by bulk kinetics.
Key Determinants Influencing K₁ Values in Enzymes
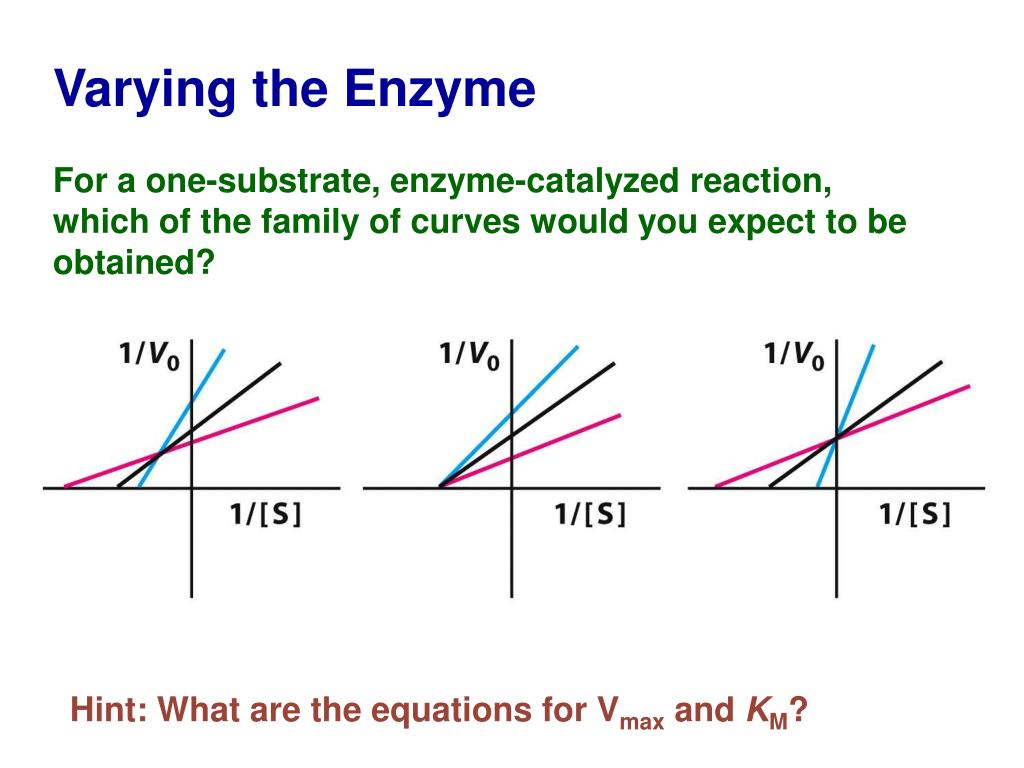
Several factors govern K₁, collectively shaping an enzyme’s binding characteristics. Molecular structure lies at the foundation: active site geometry, amino acid composition, and conformational flexibility all modulate substrate affinity.Enzymes with highly complementary binding pockets exhibit stronger associations, reflected in lower K₁ values—indicating tighter substrate grip. For example, ribonuclease A demonstrates pronounced substrate specificity due to its precisely sculpted binding cleft, resulting in low K₁ and rapid catalysis. Environmental conditions further regulate K₁.
Temperature accelerates molecular motion, sometimes enhancing binding but risking denaturation. Extreme pH disrupts charge interactions critical for substrate orientation, typically increasing K₁ as binding weakens. Cofactors and metal ions often stabilize the enzyme-substrate complex; many metalloenzymes rely on coordinated ions to intensify binding, lowering K₁ and boosting catalytic efficiency in metabolic pathways.
Allosteric regulation introduces another layer of complexity. Effectors binding at sites distinct from the active site induce conformational shifts that alter substrate affinity—sometimes reducing K₁ to dampen activity or increasing it to enhance processivity. This dynamic modulation is central to metabolic control, enabling cells to fine-tune enzyme function in response to real-time needs.
Practical Applications and Real-World Impact
K₁ enzyme kinetics extends far beyond theoretical interest—its applications permeate fields as diverse as medicine, biotechnology, and environmental science. In pharmaceuticals, identifying compounds that elevate K₁ affinity allows researchers to design potent enzyme inhibitors. HIV protease inhibitors, for instance, exploit tight substrate binding to competitively block viral replication, with K₁ data guiding lead optimization.Industrial biocatalysis leverages K₁ insights to engineer enzymes for large-scale processes. In biofuel production, cellulases with low K₁ values efficiently bind and cleave plant biomass even at sparse substrate levels, enhancing yield. Similarly, in food technology, proteases used in cheese ripening are selected based on kinetic profiles to ensure consistent texture and flavor development.
Diagnostic tools also benefit from K₁ analysis. Lactate dehydrogenase (LDH) assays, used to detect tissue infarction, rely on the enzyme’s affinity for pyruvate—modulated by K₁—to interpret blood test results with high specificity. In research, K₁ measurements validate enzyme characterization, ensuring reliable data in gene expression studies and metabolic modeling.
Cutting-edge developments include the use of machine learning to predict K₁ values from enzyme structures, accelerating drug discovery and synthetic biology workflows. These tools enable high-throughput screening of engineered enzymes, rapidly identifying variants with optimized binding properties.
The Future of K₁ Kinetics: From Research to Revolutionary Applications
K₁ enzyme kinetics stands at the intersection of fundamental biochemistry and transformative innovation.As analytical techniques grow more refined and computational models more predictive, the precision with which we quantify enzyme-substrate interactions reaches unprecedented levels. This evolution is not merely academic—each K₁ value uncovered reflects a deeper mastery over life’s catalysts, with tangible benefits across medicine, industry, and sustainability. Whether optimizing drug candidates, refining industrial enzymes, or unraveling metabolic regulation, K₁ remains an indispensable metric.
Its ability to reveal hidden dimensions of binding affinity underscores why enzyme kinetics continues to illuminate the microscopic engine driving biological function. As scientists push the boundaries of what’s possible with engineered enzymes, K₁ stands as a silent yet powerful guide—proof that the smallest molecular interactions shape the largest processes in life.
Related Post

How Nick Hogan Built a Billion-Dollar Empire: The Untold Story Behind His Net Worth
Juliet Litman The Ringer Bio Wiki Age Height Husband Bachelor Party Salary and Net Worth
<i>IoTeX AI Price Predictions: Décrypter Les Prévisions Crypto avec Précision</i>
Sza's Children: Setting the Record Straight

