Unlocking Life’s Energy: The Precise Equation Behind Cellular Respiration
Unlocking Life’s Energy: The Precise Equation Behind Cellular Respiration
At the very core of cellular metabolism lies a powerful biochemical transformation: the conversion of nutrients into usable energy, enabling every function from muscle contraction to neural signaling. This process, cellular respiration, unfolds through a meticulously orchestrated series of reactions governed by a single, elegant chemical equation. Understanding the full equation—expressed through precise chemical formulas—and the steps that follow reveals not just how cells generate ATP, but how life sustains itself at the molecular level.
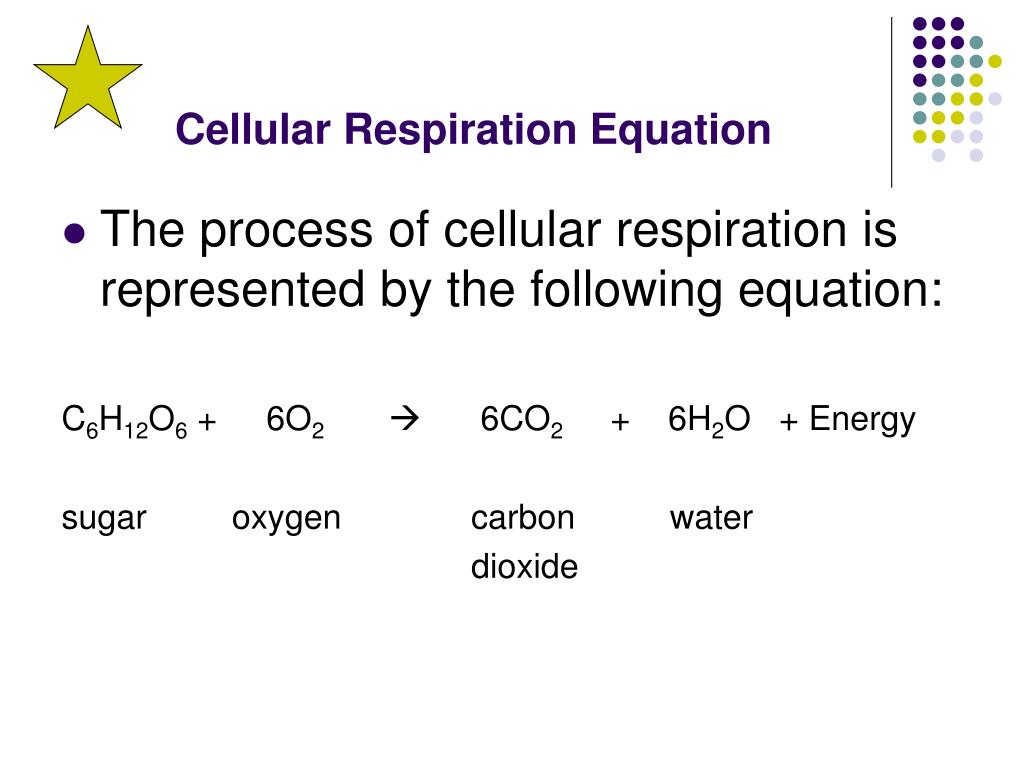
The equation, in its most comprehensive form, captures this energetic revolution with remarkable clarity, forming the foundation of bioenergetics. The central equation for cellular respiration is:
C6H12O6 + 6O2 → 6CO2 + 6H₂O + Energy (ATP)
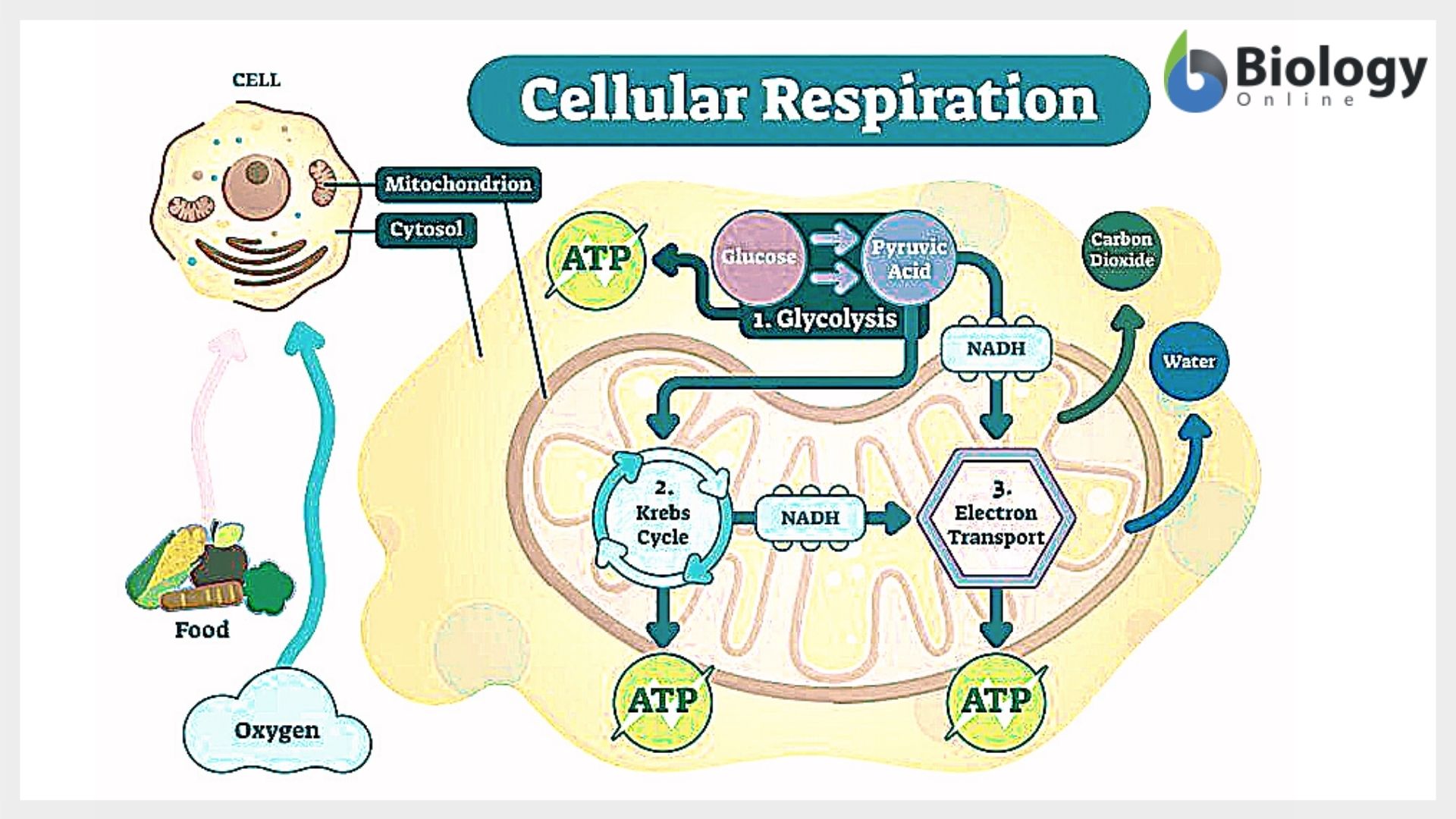
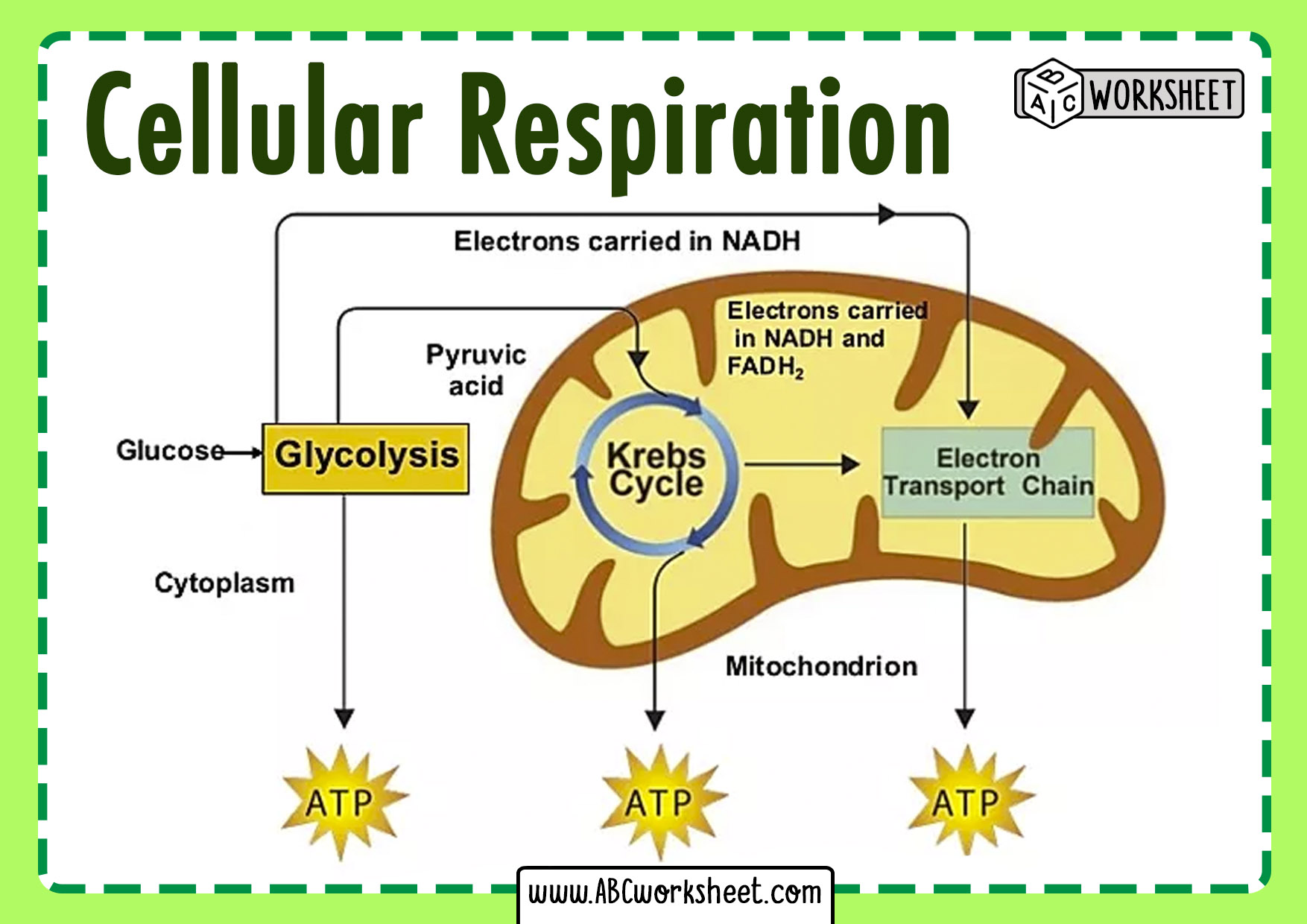
This balanced representation encapsulates the complete transformation of glucose (C₆H₁₂O₆), a six-carbon sugar, and oxygen (O₂) into carbon dioxide (CO₂), water (H₂O), and energy stored primarily as adenosine triphosphate (ATP). The stoichiometry reflects a precise ratio: one molecule of glucose reacts entirely with six molecules of oxygen, producing six carbon dioxide molecules and six water molecules, releasing enough energy to drive cellular processes.But behind this simple equation lies a multi-stage metabolic journey involving glycolysis, the Krebs cycle, and the electron transport chain—each stage carefully coordinated and energy-preserving. The transformation begins with glycolysis, a fragile eight-step process occurring in the cytoplasm that breaks down one glucose molecule. Though it yields only a net gain of two ATP molecules and two NADH, its role extends beyond direct energy production.
It primes glucose into two pyruvate molecules while generating critical electron carriers essential for downstream energy extraction. “Glycolysis is not just a first step—it is the gateway to aerobic efficiency,” notes Dr. Elena Torres, a biochemist at the Institute for Molecular Metabolism.
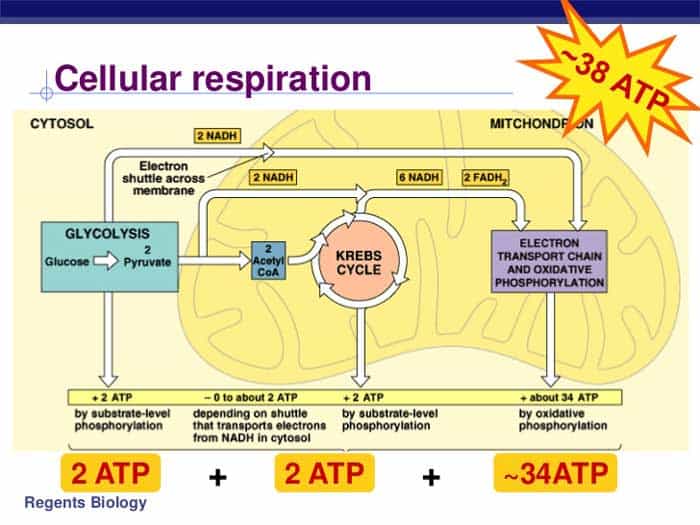
“Without it, the complex electron transfer in oxidative phosphorylation would lack the redox fuel to initiate.” As pyruvate enters the mitochondria, it undergoes oxidation in the Krebs cycle (or citric acid cycle), a tightly regulated sequence where carbon atoms are systematically removed and transferred to carrier molecules. Each acetyl-CoA derived from glucose contributes—via three NADH, one FADH₂, and one GTP per turn—feeding into the next energy-conserving phase. The Krebs cycle itself closes the metabolic loop by regenerating oxaloacetate, preparing for the next influx of acetyl units.
“This cycle is nature’s reactor: compact, efficient, and remarkably high-yielding,” explains Dr. Marcus Lin, an expert in intermediary metabolism. “One glucose molecule yields eight NADH, two FADH₂, and four ATP—and the real power lies in what follows.” The エネルギーの最終源をloaded in the Elektronentransportkette wirkung entfaltet sich: Protonen werden über die mitochondriale Innenmembran gepumpt, um elektrische Gradienten aufzubauen; dieser Energieunterschied treibt die Synthese von bis zu 26–28 ATP molecules via oxidative phosphorylation.
The full equation sums these outputs, but the real marvel lies in the chemical details: the oxidation of organic bonds releases electrons, which flow through protein complexes, releasing energy to pump protons across the membrane. Oxygen acts as the terminal electron acceptor, combining with electrons and protons to form water—a critical step that keeps the chain moving. Chemically, each NADH delivers two high-energy electrons that traverse cytochromes, releasing enough energy to pump approximately three protons across the membrane.
FADH₂ contributes two electrons with less energy, contributing to fewer protons pumped per molecule. The resulting proton-motive force powers ATP synthase, a molecular turbine that catalyzes the phosphorylation of ADP to ATP. “This electron cascade is nature’s most efficient miniaturized power plant,” remarks Dr.
lines between porosity and power. The equation, therefore, is not just a static formula but a dynamic snapshot of redox chemistry, electron flux, and coupling of chemical energy to mechanical ATP synthesis. Beyond ATP, cellular respiration yields water—visually tangible confirmation of carbon and oxygen transformation—and releases heat as a byproduct, playing a vital thermoregulatory role.
While the equation emphasizes glucose and oxygen, in reality, most cells rely on fatty acids and amino acids, each feeding into the same metabolic framework. The universality of this equation across aerobic organisms—from bacteria to humans—salutes the evolutionary conservation of energy transformation. In every cell, whether in a squirrel’s muscle or a neuron’s synapse, this single chemical equation powers existence untold times.
Understanding cellular respiration through its chemical equation offers more than stoichiometric insight—it reveals how biological systems harness and regulate energy with quantum-level precision. Every atom, every electron, every bond contributes to the sustained engine of life. In mastering WhatIsTheEquationForCellularRespirationUsingChemicalFormulas, we grasp the elegance of nature’s design: energy not given, but shaped—meticulously, reliably, and beautifully—from the carbon in a meal into the lifeblood of biological function.
Related Post
Who Was Tucker Carlsons First Wife Unveiling the Private Life of a Media Titan
FaZe Adapt Net Worth and Earnings
The ZIP Code of Carson City: Gateway to Nevada’s Capital

David Muir’s Partner: Unveiling the Personal and Professional Life of a Renowned Journalist

