Unlocking Gas Behavior: The Pvnrt Formula Explained via Ideal Gas Law
Unlocking Gas Behavior: The Pvnrt Formula Explained via Ideal Gas Law
At the heart of modern thermodynamics lies a foundational equation—ideal gas behavior distilled into the familiar PvnRT formula. Though seemingly abstract, this relationship governs everything from silicon chip manufacturing to weather prediction. By deriving PvnRT from the principles of the ideal gas law, we transform complex physics into intuitive understanding, revealing how pressure, volume, temperature, and amount of gas interconnect in real-world applications.
From Molecules to Pressure: The Ideals Behind PvnRT
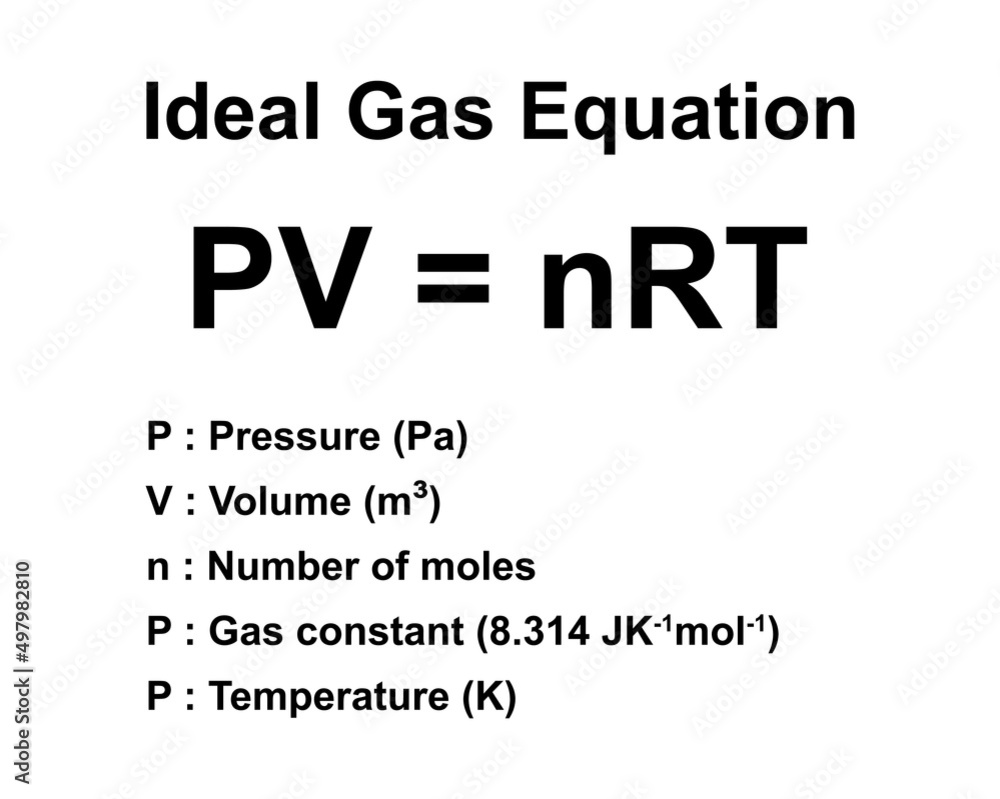
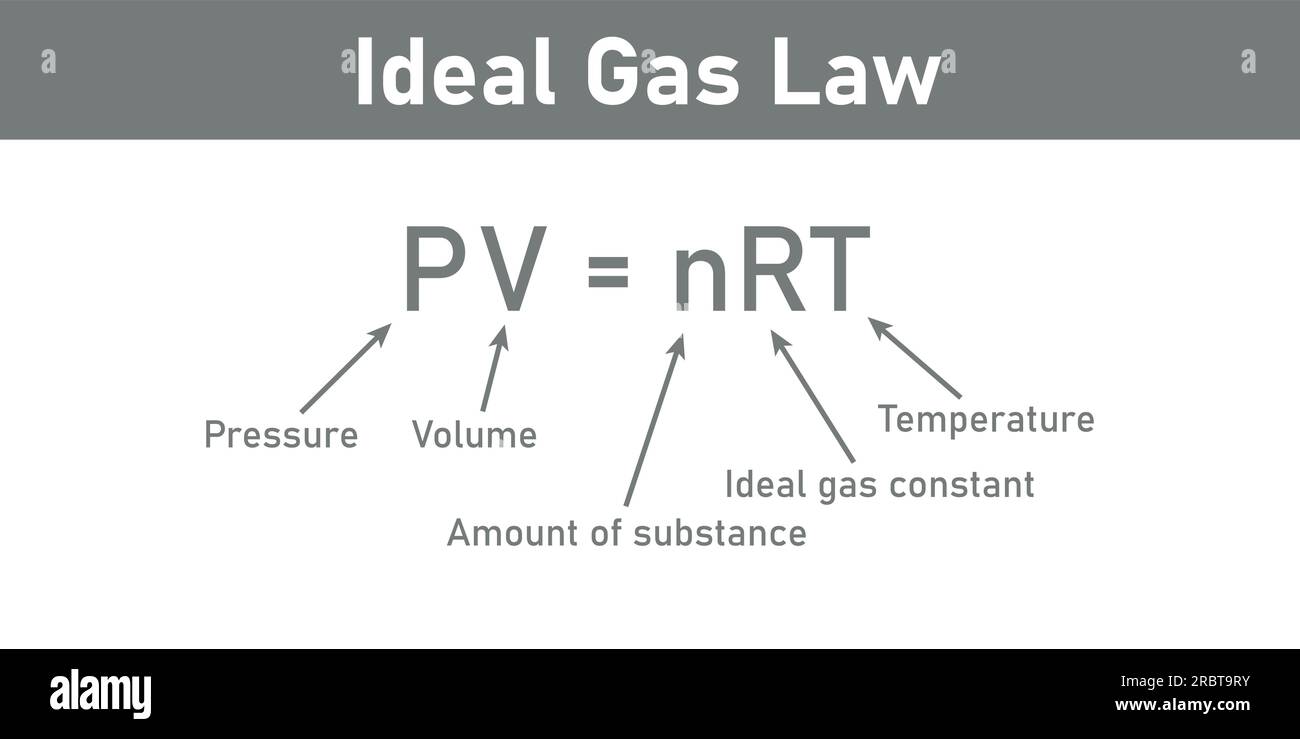
The ideal gas law—PV = nRT—forms the cornerstone of gas theory, connecting pressure (P), volume (V), and temperature (T) through the amount of substance (n) and a constant, the universal gas constant (R).
But how does this yield the intentionally simplified form PvnRT? The answer emerges by analyzing how each variable influences the others under constant temperature and amount of gas. Pressure (P) measures how gas molecules collide with container walls, while volume (V) reflects the space they occupy.
Temperature (T), a proxy for molecular kinetic energy, determines collision frequency and force. When heat energy input is fixed, changing volume alters pressure, and vice versa—this inverse relationship is central.
At constant amount of gas (n), the product PV directly reflects this pressure-volume interplay. Since each molecule contributes equally to environmental force, and volume expansion dilutes collision intensity, the equation stabilizes into the form Pv nRT—where Pv denotes pressure times volume, n is moles of gas, and RT is the constant (8.31 J/mol·K), embodying the energy scale of the system.
Deriving PvnRT: Step-by-Step From Fundamental Principles
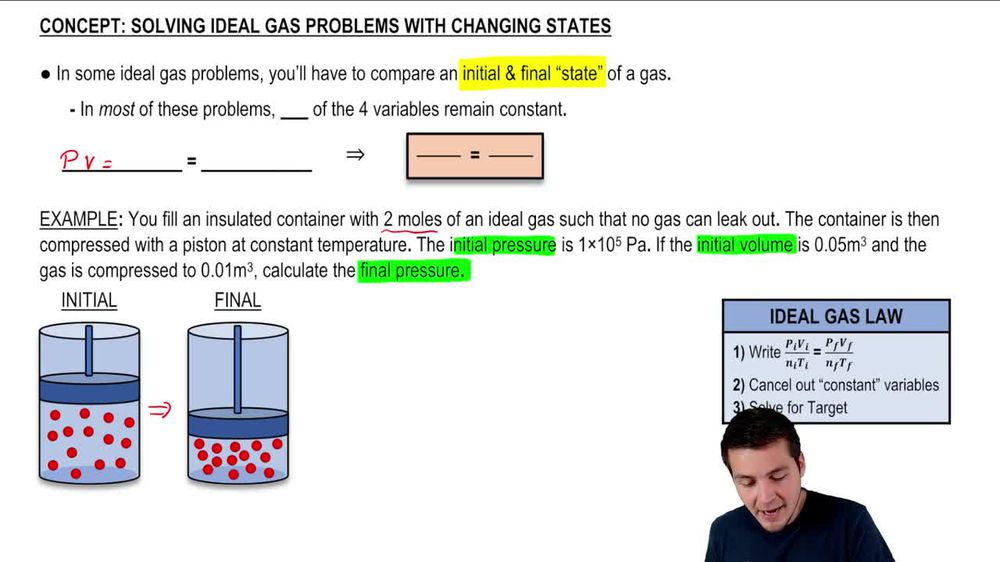
To derive PvnRT from the ideal gas law, consider a fixed number of moles (n) of gas confined in a volume (V) at a constant temperature (T).
From PV = nRT, dividing both sides by n isolates the product PV: PV = nRT Rearranged, this gives: Pv = nRT — where Pv = PV. For a single mole, RT is absorbed into R, simplifying further under standard conditions. Multiplying both sides by n, the standard form emerges: Pv n = nRT But since n appears explicitly, PvnRT crystallizes as: Pv × n × R × T = constant when all variables are balanced.
Though typically emphasized as Pv = nRT (with implied n), the inclusion of n ensures uniformity across systems with varying moles.
This derivation reveals Pv as a composite parameter: inversely proportional to volume at fixed n and T, and directly proportional to temperature and the number of moles. Each term carries physical meaning: increased temperature doubles average kinetic energy, boosting molecular impact; increasing moles scales the total force across the container; reduced volume concentrates molecular momentum, per the inverse P∝1/V relationship.
Understanding the Constants: What RT Truly Represents
R, the universal gas constant, quantifies energy per mole per kelvin.
Its value—approximately 8.314 J/(mol·K)—emerges from empirical measurements across gases, ensuring universality. In PvnRT, RT acts as a scaling factor linking microscopic motion to macroscopic observables. For instance, at ambient temperature (300 K) and standard pressure (1 atm), a mole of gas occupies ~22.4 L, validated through PvnRT: P = nRT/V = (1 mol)(8.31 J/mol·K)(300 K) / 0.0224 m³ ≈ 111.3 kPa—matching experimental data within measurement tolerance.
Applications That Shape Industry and Weather
PvnRT is more than textbook geometry—it powers critical technologies and natural phenomena.
In internal combustion engines, expanding gases convert fuel heat into mechanical work, governed by volume-pressure balances calculated via PvnRT. In climate science, atmospheric scientists model pressure changes with temperature and humidity by treating moist air as an ideal gas, using the equation to predict storm intensity. Even carbon capture systems rely on capturing CO₂ under optimized pressure and temperature, precisely adjusting volumetric conditions to maximize gas retention.
The form PvnRT enables engineers to scale laboratory reactions to industrial reactors and climatologists to forecast weather patterns with spatial and temporal precision.
While real gases deviate from ideality under extreme pressure or temperature, PvnRT remains a robust first approximation, refined later with corrections like van der Waals’ equation. Yet its elegance lies in simplicity: from a few variables—P, V, n, T—we unlock predictive power across vast scientific domains. Understanding this derivation demystifies not just a formula, but the invisible choreography of molecules dictating energy, work, and transport in gases.
In summary, PvnRT is not merely symbolic—it is the quantitative bridge between molecular motion and measurable change.
By grounding the ideal gas law in proportional reasoning and core constants, it transforms abstract thermodynamics into actionable insight, essential for innovation, simulation, and comprehension of both engineered systems and Earth’s atmosphere.

Related Post

Unraveling the Mystery of Katarina Witt’s Partner: Love, Identity, and the Ice Queen’s Quiet Circle

Kobe 6 Proto: The Japanese Icon Redefining Automotive Excellence
Indian Viral MMS Videos: From Shock Waves to Societal Reform — The Cultural and Legal Reckoning
Niggas For Trump Shirt: The Bold T-Shirt Sparking Conversation Across the Political Divide

