Unlock the Geometry of Chemistry: How PhET’s Molecule Shapes Transforms Understanding
Unlock the Geometry of Chemistry: How PhET’s Molecule Shapes Transforms Understanding
At the heart of chemistry lies an invisible yet powerful language—molecular geometry—the three-dimensional arrangement of atoms that dictates how molecules behave, react, and interact. Molecule Shapes on PhET Interactive Simulations offers learners a dynamic, visual gateway to mastering this foundational concept. By translating abstract atomic coordinates into intuitive, manipulable models, the platform enables students, educators, and enthusiasts alike to explore geometries that govern chemical properties and reactivity.
With real-time adjustments and interactive feedback, the simulation turns complex theory into tangible discovery, revealing why molecules like methane bend or why water’s bent shape fuels its pivotal role in life.
PhET’s Molecule Shapes simulation is more than a visual aid—it’s a transformative learning tool that demystifies VSEPR theory. The VSEPR model, which explains molecular geometry through electron pair repulsion, becomes accessible as users rotate, resize, and inspect molecules like carbon dioxide (linear), ammonia (trigonal pyramidal), and sulfur tetrafluoride (square planar).
By interacting directly with molecular structures, learners grasp why electron domains—both bonding and lone pairs—shape the final outline of molecules. Each configuration is rooted in precise spatial logic, empowering users to predict and explain reactivity, polarity, and intermolecular forces.
The Science Behind Molecular Shapes: VSEPR Theory in Action
Central to the Molecule Shapes simulation is Pauling’s VSEPR (Valence Shell Electron Pair Repulsion) theory, a cornerstone of modern chemistry education. VSEPR posits that electron pairs around a central atom arrange themselves to minimize repulsion, resulting in predictable geometries.Physics and chemistry converge here: just as atoms repel each other in space, electron pairs—whether involved in bonds or existing as lone pairs—exert forces that determine shape.
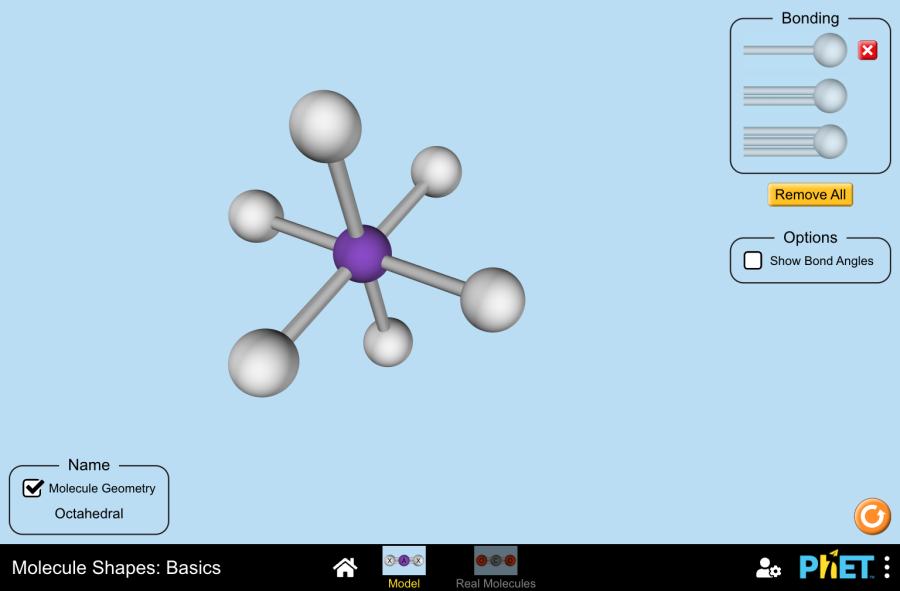
The simulation rigorously applies this principle by modeling electron domains and their role in shaping molecules. Each interactive model displays: - The central atom, surrounding by bonded atoms and lone pairs - Angles between bonds, quantifiable through real-time measurement tools - Relationships between geometry and electron count - Dynamic adjustments showing how lone pairs compress bond angles For example, a water molecule (H₂O) appears bent (V-shaped), not linear, despite having four electron domains.
Two bonds and two lone pairs force a 104.5° angle—smaller than tetrahedral water’s 109.5° due to stronger lone pair repulsion, a nuance vividly illustrated in PhET’s interface.
Exploring Key Molecular Geometries Through the Simulation
PhET’s Molecule Shapes platform enables deep exploration of core molecular forms, each tied to distinct electronic configurations: - **Linear (180° bond angles)**: Molecules like CO₂ or HCN showcase this symmetry when two bonding pairs surround a central atom with no lone pairs. Users observe how the straight line minimizes repulsion between equivalent electron domains.- **Trigonal Planar (120° angles)**: Benzene (C₆H₆) and sulfur tetrafluoride (SF₄) illustrate this plane geometry, where electron pairs settle in a flat arrangement. The simulation highlights how this flat shape influences molecular stability and symmetry. - **Tetrahedral (109.5°)**: Family of CH₄, NH₃, and CCl₄ demonstrates a four-bonded central atom expanding around four electron domains.
While ideal tetrahedral angles are exactly 109.5°, the presence of lone pairs—such as in ammonia—distorts symmetry, reducing angles and introducing polarity. - **Polar and Nonpolar Molecules**: The tool clarifies how geometry interacts with electronegativity. Polar molecules like H₂O and SO₂ arise from asymmetric shapes and polar bonds, while symmetric geometries—like in CO₂ or SF₆—often result in nonpolarity despite individual bond polarity.
Interactive features such as bond angle measurement, lone pair visualization, and comparison tools allow learners to test hypotheses—what happens if a lone pair replaces a bond? How do expanded octets in molecules like PF₅ alter expected shapes?
Interactive Learning: How Students and Educators Harness the Simulation
For students, Molecule Shapes is not passive observation—it’s active inquiry. By manipulating models and measuring angles, learners develop spatial reasoning, a critical skill in chemistry and related STEM fields.Educators use the platform to guide inquiry-based lessons: devising experiments to verify geometries, compare theoretical vs. observed behavior, or explore exceptions like hypervalent molecules. Classroom applications extend beyond abstract theory: - **Hypothesis Testing**: Students predict shapes based on electron counts, then verify with the simulation.
- Real-Time Feedback: Instant measurement tools confirm correct geometry, reinforcing accurate mental models. - **Collaborative Learning: Shared browser access enables group exploration and peer teaching, deepening conceptual mastery. - **Bridging Theory and Application: Each shape connects directly to chemical properties—why water boils at a higher temperature than H₂S, or why noble gas compounds exhibit unexpected geometries.
PhET’s intuitive interface ensures accessibility across skill levels, making the otherwise complex VSEPR model comprehensible without sacrificing scientific rigor. As one learner notes, “Seeing atoms physically rotate and repel visually was like magic—it made the ‘why’ of molecular shapes real.”
The Broader Impact on Science Literacy and STEM Success
Mastering molecular geometry shapes a learner’s scientific worldview. It reveals chemistry not as a stack of facts, but as a coherent system governed by physical principles.PhET’s simulation turns conceptual barriers into opportunities—students who engage with the tool develop confidence, intuition, and critical thinking skills essential for advanced study. Why molecular shapes matter beyond the classroom: - Enable predictions of physical properties: boiling points, polarity, solubility. - Clarify reactivity patterns in organic and inorganic chemistry.
- Support understanding of biological macromolecules, where geometry dict



Related Post

Unlocking the Neural Pathways: How Dorsal Rami Function Drives Precision in Sensory and Motor Control
-1625732021738.jpg)
Is Dina Meyer Married? Unveiling the Truth Behind Her Relationship Status
Towanda Braxton All you need to know about this American celebrity

Track Hurricane Erin in Real Time with the Latest Tracker Map – What You Need to Know

