Trigonal Pyramidal Molecule: The Shaped Mystery Behind Molecular Geometry
Trigonal Pyramidal Molecule: The Shaped Mystery Behind Molecular Geometry
At the heart of modern chemistry lies a subtle yet profound structural archetype: the trigonal pyramidal molecule. Defined by a central atom bonded to three surrounding atoms or lone pairs, with a distinct three-dimensional pyramid shape, this geometry governs molecular polarity, reactivity, and biological function. From ammonia to complex pharmaceuticals, the trigonal pyramidal form reveals itself as a fundamental building block of chemical behavior—shaping everything from atmospheric dynamics to essential biochemical pathways.
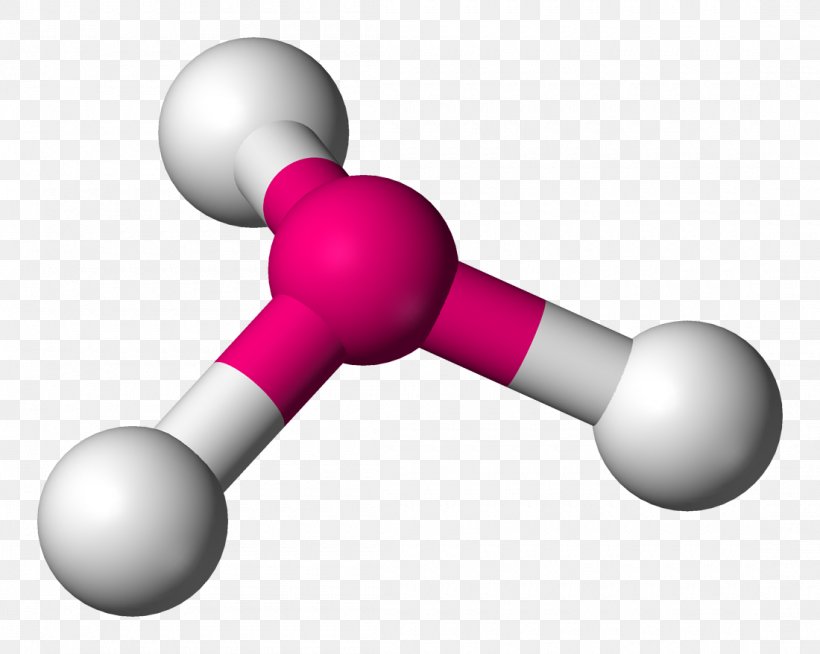
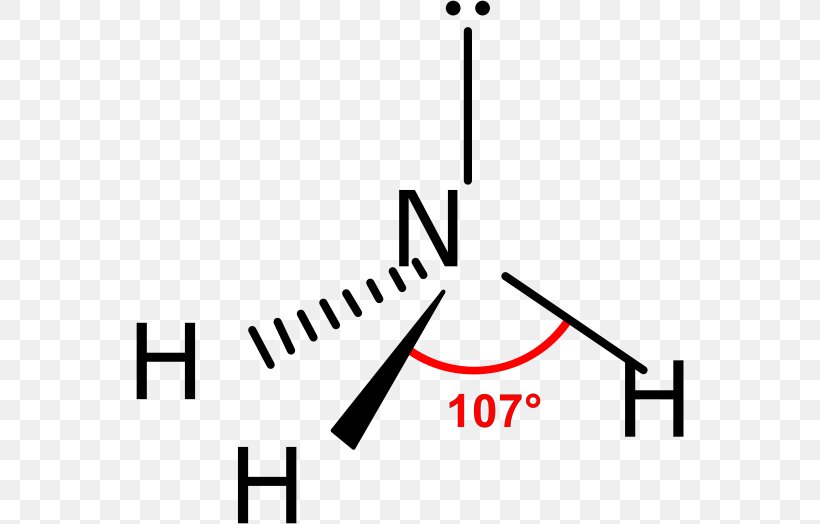
The foundation of trigonal pyramidal geometry arises from the electron domain theory, specifically the Valence Shell Electron Pair Repulsion (VSEPR) model.According to VSEPR, electron pairs around a central atom arrange themselves to minimize repulsion. In the trigonal pyramidal case, four electron domains exist—three bonding pairs and one lone pair—grouping around the central atom in a tetrahedral arrangement. However, because the lone pair exerts stronger repulsion than bonding pairs, it pushes the three bonded atoms closer together, creating a pyramid with a triangular base and a apex pointing upward.
This geometric distortion is pivotal: it determines molecule polarity, influencing solubility, phase behavior, and intermolecular interactions.
The classic example of this structure is ammonia (NH₃), where nitrogen—despite having five valence electrons—forms three covalent bonds with hydrogen atoms and retains one lone pair. Its trigonal pyramidal shape gives NH₃ a net dipole moment, making it highly soluble in water and a key player in acid-base chemistry: “Ammonia’s lone pair allows it to readily accept protons, a behavior defined by its unique geometry,” explains Dr. Elena Marquez, a physical chemist at the Institute for Molecular Structure.
Beyond ammonia, several biologically and industrially relevant molecules adopt this geometry.
Methylamine (CH₃NH₂), an important amine in herbicides and gas tratements, exhibits a similarly pyramid-shaped nitrogen center, enabling it to act as a nucleophile in organic synthesis. In pharmaceuticals, the trigonal pyramidal motif appears in drug candidates where nitrogen lone pairs facilitate binding to enzyme active sites, enhancing specificity. For instance, certain antidepressants and antiviral agents rely on substitution patterns around pyramidal carbons to achieve optimal pharmacokinetic profiles.
What sets trigonal pyramidal molecules apart is their responsiveness to environmental conditions.
Lone pairs increase molecular polarity, often leading to stronger hydrogen bonding capacity—critical in biological systems. In aqueous solutions, trigonal pyramidal amines like ammonia reduce pH through proton donation, functioning efficiently as weak bases. “Their electron-rich lone pairs make them excellent proton scavengers,” notes Professor Rajiv Nair, an expert in molecular polarity.
“This behavior underpins key processes in cellular metabolism and atmospheric chemistry.”
Synthetic chemists exploit trigonal pyramidal geometries to design tailored molecules. By introducing substituents—alkyl, aryl, or functional groups—researchers modulate steric and electronic effects, directing reactivity and selectivity. In catalysis, pyramidal metal complexes (e.g., certain transition-metal amides) leverage lone pair stability to activate small molecules like N₂ or CO₂, offering greener routes to valuable chemicals.
The precision enabled by controlling molecular shape translates into smarter, more efficient industrial processes.
Despite their prevalence, trigonal pyramidal molecules pose subtle analytical challenges. Their dipole moments and hydrogen-bonding tendencies affect spectroscopic signatures—making NMR, IR, and X-ray diffraction interpreting both informative and complex. “Urgent work in stereochemical modeling focuses on predicting how pyramidal distortions impact molecular dynamics,” observes Dr.
Marquez. “Accurate descriptors are essential for drug discovery and materials science.”
In materials science, geometric control over pyramidal configurations opens new frontiers. Pyramidal termini on nanoparticles or organic frameworks influence surface reactivity, catalytic activity, and selectivity in molecular sieving.
Surface-functionalized pyramid-shaped ligands enable selective adsorption, promising advances in gas storage and separation technologies. The ability to engineer molecular geometry at the atomic scale transforms fundamental principles into cutting-edge applications.
But challenges remain in mastering behave. Lone pair interactions
Related Post
B6 73: The Little-Overlooked Bioactive Compound Reshaping Health Sciences
Army Of Soldiers: Unveiling the Structure, Roles, and Impact of Modern Military Forces

