The Molecular Puzzle of Nitrogen Dioxide: Decoding Its Lewis Structure and Chemical Significance
The Molecular Puzzle of Nitrogen Dioxide: Decoding Its Lewis Structure and Chemical Significance
Nitrogen dioxide, a colorless gas with a distinctive sharp odor, plays a pivotal role in atmospheric chemistry and environmental science—yet its molecular structure reveals a subtle complexity that defies simplistic interpretation. The Lewis structure of nitrogen dioxide (NO₂) serves not only as a foundational blueprint for understanding its bonding but also as a gateway to grasping its reactivity, environmental impact, and role in biological systems. At the heart of this molecule lies a dynamic interplay between nitrogen and oxygen atoms, shaped by uneven electron sharing and resonance, which defines its chemical behavior in unexpected ways.
The Core of the Molecule: Bonding in Nitrogen Dioxide
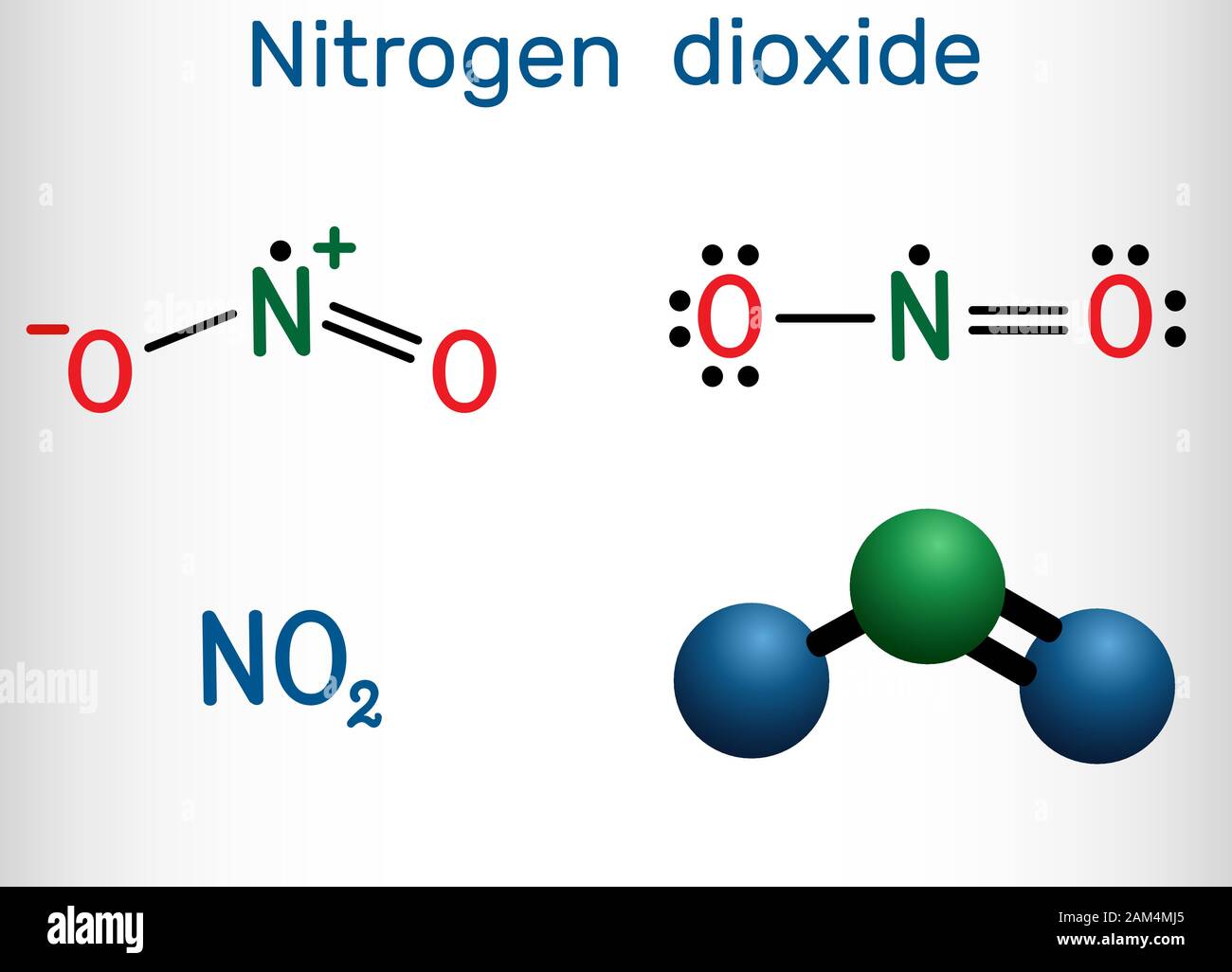
Nitrogen dioxide is composed of one nitrogen atom covalently bonded to two oxygen atoms, forming a bent, asymmetric molecule with a central nitrogen surrounded by two unpaired electrons.
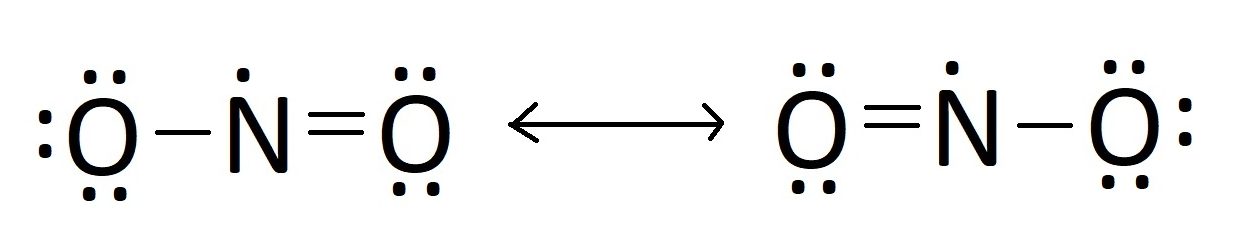
Unlike diatomic molecules, NO₂ lacks a clear octet due to nitrogen’s ability to expand its valence shell beyond eight electrons, a consequence of its position in the third period. The Lewis structure of NO₂ reflects this unusual electron distribution: one nitrogen-oxygen double bond and one nitrogen-oxygen single bond, with one unpaired electron residing on nitrogen—making the molecule a free radical.
The canonical Lewis structure displays a primary resonance hybrid: a core with a double bond between nitrogen and one oxygen, and a single bond and a lone pair on nitrogen, accompanied by an unpaired electron. This resonance stabilizes the molecule but also amplifies its reactivity.
According to chemists at atmospheric research institutions, “NO₂’s structure is paradoxical—electron-rich enough to participate actively in oxidation reactions, yet unstable enough to continuously shift its electron distribution, defending its radical character.”
Resonance and Electron Delocalization: Why NO₂ Isn’t Just What It Seems
Understanding NO₂’s behavior requires a deeper dive into resonance. In reality, the molecule exists not as a single rigid structure but as a hybrid of two major resonance forms: one with a double bond to the left oxygen and a single to the right, and another with the double bond localized to the right oxygen. This delocalization of electrons across both O–N bonds explains the molecule’s partial double-bond character and contributes to its high oxidation potential.
Each resonance form stabilizes the positive and negative charges partially, allowing NO₂ to act as both a weak acid and a strong oxidizing agent.
This dual nature enables NO₂ to react vigorously in aqueous environments—dissolving in rainwater to form nitric acid (HNO₃), a key player in acid rain formation. Environmental scientists emphasize, “The resonance stabilization of NO₂ is central to its transformation in the atmosphere, where it participated in chain reactions that degrade ozone and contribute to smog.”
Environmental and Biological Impacts: From Air Pollution to Cellular Stress
Nitrogen dioxide’s molecular characteristics directly influence its role in environmental degradation. When emitted primarily from combustion processes—factories, vehicles, and power plants—NO₂ is a primary precursor to ground-level ozone, a hazardous air pollutant.
It reacts with volatile organic compounds in sunlight to form photochemical smog, reducing air quality and posing serious respiratory risks. Public health studies confirm elevated NO₂ levels correlate with increased asthma incidence and lung inflammation, particularly in urban centers.
Biologically, NO₂’s reactivity extends into human tissue. Inside the respiratory tract, it undergoes rapid decomposition upon contact with moisture, generating reactive oxygen species (ROS) and nitric acid.
“The same resonance that fuels NO₂’s environmental vigor makes it a double-edged sword in biology—it signals danger but also triggers cellular defense mechanisms,” explains a toxicologist specializing in airborne pollutants. Chronic exposure damages ciliated epithelial cells, weakening natural filtration barriers and heightening susceptibility to infection.
Engineering Insights: Why Nitrogen Dioxide Resists Simple Representation
From a structural chemistry perspective, NO₂ defies static representation. Its bent geometry—with an O–N–O angle near 134 degrees—arises from sp² hybridization of nitrogen and lone-pair repulsion, while the radical nitrogen center remains in a high-energy state.
Spectroscopic and computational studies reveal that NO₂’s actual electronic structure involves fluctuating bond orders, with experimental evidence supporting the resonance hybrid model over fixed bonds.
These insights challenge traditional Lewis structures that depict discrete bonds. Modern computational chemistry uses molecular orbital theory to more accurately model electron density, showing NO₂’s bonding is inherently dynamic. “Attempting to fix NO₂’s structure into rigid lines obscures the very reactivity that makes it so significant,” notes a quantum chemist.
Analysts stress that understanding this electronic flexibility is key to developing accurate environmental models and targeted pollution control strategies.
The Broader Significance: Why Mastering NO₂’s Lewis Structure Matters
The nitrogen dioxide molecule, with its intricate electron dance and dual reactivity, stands as a testament to the hidden complexity in seemingly simple chemical systems. Its Lewis structure is not merely a static diagram—it reflects a dynamic, resonance-stabilized entity with profound implications across atmospheric science, environmental engineering, and human health. Recognizing NO₂’s full structural narrative empowers scientists to predict its behavior with greater precision, design effective mitigation technologies, and protect public well-being in an increasingly polluted world.
As research continues to unfold, NO₂ remains a critical case study in how molecular architecture shapes planetary and physiological systems alike.
“To understand nitrogen dioxide is to understand a key player in Earth’s reactive chemistry,” concludes a leading atmospheric chemist. “Its structure is fleeting, its impacts lasting—making it both a challenge and a gateway to deeper scientific insight.”

Related Post
Who Is Tyana Hansen the Australian Playboy Model
Brady Potter Age Wiki Sexuality Bio Height Boyfriend Net worth
What Every American Needs To Know About The Sabrina Banks Onlyfans Leak

