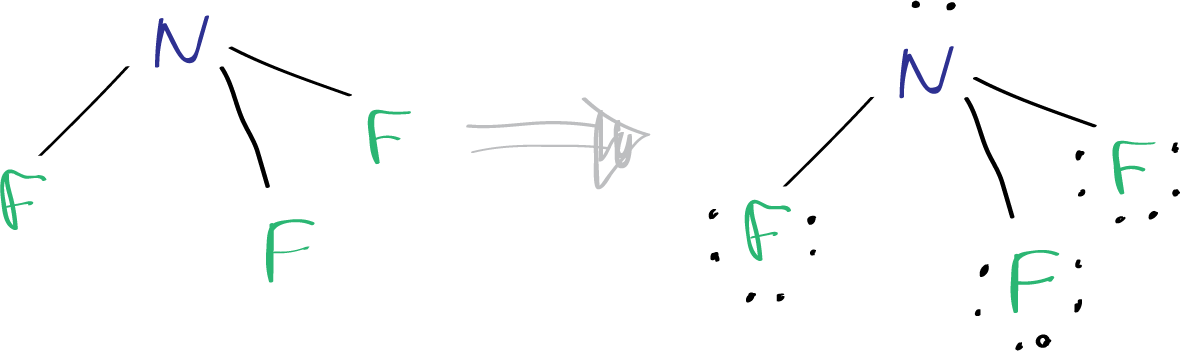The Electrical Science Behind Nf3 Lewis Structures Decoded – Why Nf3 Matters in Molecular Chemistry
The Electrical Science Behind Nf3 Lewis Structures Decoded – Why Nf3 Matters in Molecular Chemistry
In the intricate world of chemical bonding, few molecules spark as much fascination—and scientific scrutiny—as nitrogen trifluoride (Nf3), with its unique Lewis structure defying conventional norms. Though often overlooked, the Lewis model applied to Nf3 reveals critical insights about electron distribution, molecular geometry, and chemical reactivity. Understanding this structure isn’t just an academic exercise—it shapes advancements in materials science, pharmaceuticals, and environmental chemistry.
The Lewis approach, named after Gilbert N. Lewis who revolutionized electron theory, remains the cornerstone for visualizing bonding at the atomic level, even in complex cases like Nf3. By dissecting how valence electrons arrange around nitrogen and three fluorine atoms, scientists uncover why Nf3 exhibits properties that challenge traditional fluorine chemistry.
Nitrogen trifluoride presents a striking anomaly: nitrogen, with just five valence electrons, forms three strong σ-bonds with fluorine atoms, leaving residual electron density that distorts ideal tetrahedral geometry. According to the Lewis model, nitrogen typically participates in sp³ hybridization, expecting four equivalent orbitals—yet fluorine’s high electronegativity and small atomic size force a unique compromise. The central nitrogen shares one unpaired electron pair in a doughnut-shaped region, reducing symmetry and resulting in a distorted structure.
This deviation from ideal tetrahedral angles (observed at approximately 100° bookkeeping) stems from lone-pair repulsion and fluorine’s strong pull. The resulting molecular geometry—often described as see-saw or distorted tetrahedral—impacts reactivity, making Nf3 more dynamic than expected for such a stable molecule.
The Lewis structure of Nf3 follows: N bonded to three F atoms, with one lone pair residual after forming three N–F sigma bonds. Each F shares a complete octet via a covalent bond, while nitrogen completes its octet through a combination of bonding and retained electron density.
Key takeaways from this arrangement include:
- Electron Deficiency on Nitrogen: Unlike ammonia (NH₃), where nitrogen has a lone pair, Nf3’s lone pair occupies a separate orbital, leaving partial electron unavailability for additional interactions.
- Bond Length Anomalies: Fluorine’s small radius and strong polarizing power compress bonding orbitals, shortening N–F distances compared to similar molecules like NF₃, where fluorine dominates geometry.
- Thermal Instability: The residual lone electron pair introduces higher intrinsic reactivity; Nf3 tends to decompose more readily in high-energy environments, a factor critical in industrial synthesis.