The Crucial Role of Limiting Reactant in Chemical Reactions: Precision in Every Mole
The Crucial Role of Limiting Reactant in Chemical Reactions: Precision in Every Mole
In the intricate dance of chemical reactions, not all reactants play equal parts—some are the unsung gatekeepers that determine the outcome. At the heart of this control lies the concept of the limiting reactant: the substance that runs out first, capping the maximum amount of product formed. Without understanding limiting reactants, scientists risk miscalculating yields, wasting resources, and undermining experimental integrity.
From industrial synthesis to classroom chemistry, this principle governs efficiency, cost, and safety.
Limiting reactant definition refers to the reactant in a chemical equation that is fully consumed before any other, thereby restricting the extent to which the reaction can proceed. Once depleted, even an excess of other reactants remains unreacted, determining the theoretical maximum of product that can be generated.
This concept is indispensable in stoichiometry—the science of quantitative relationships between reactants and products.
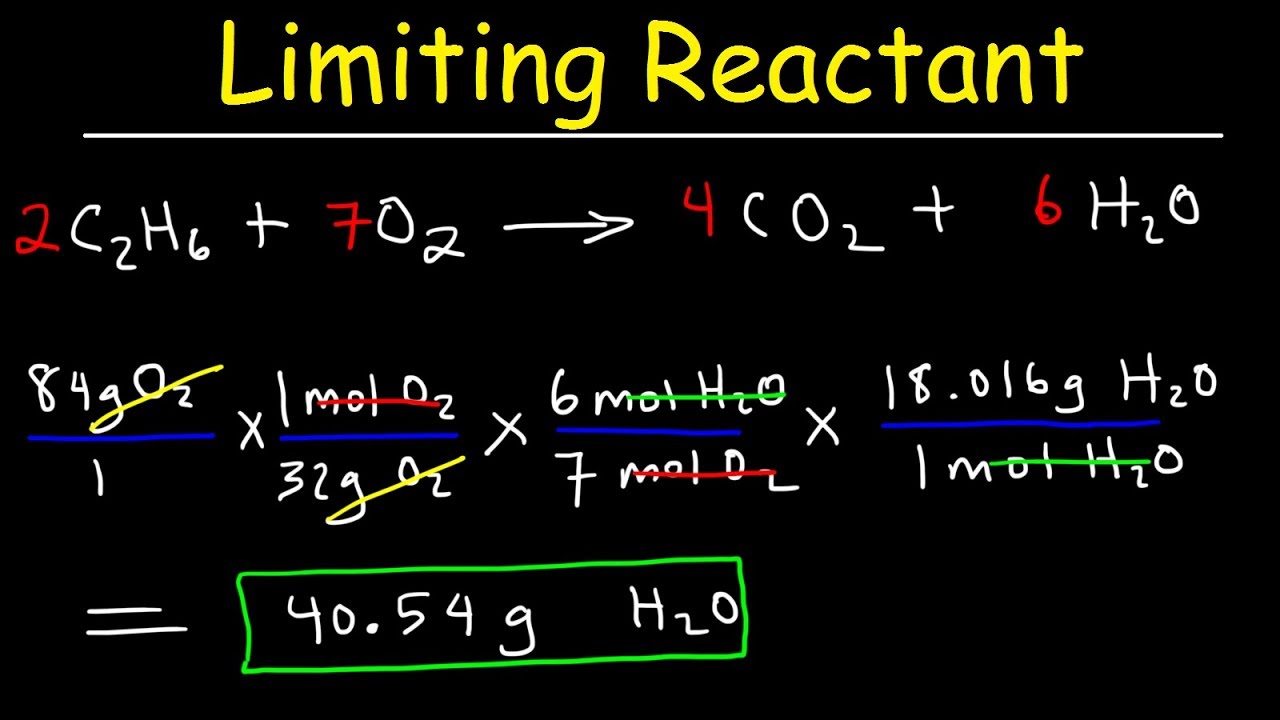
In practical terms, identifying the limiting reactant is essential for reliable calculations. Consider a simple synthesis reaction: 2A + 3B → C + D. If 4 moles of A react with 6 moles of B, while the balanced equation shows A requires 1.5× as much B per mole to fully react, the ratio reveals A as the limiting agent.
Only 4 moles of A (posing a demand of 8 moles of B at 2:3, but only 6 available) halt the reaction. This disparity highlights how limiting reactants cap reaction规模 and effectiveness.
Understanding the Stoichiometric Basis of Limiting Reactants
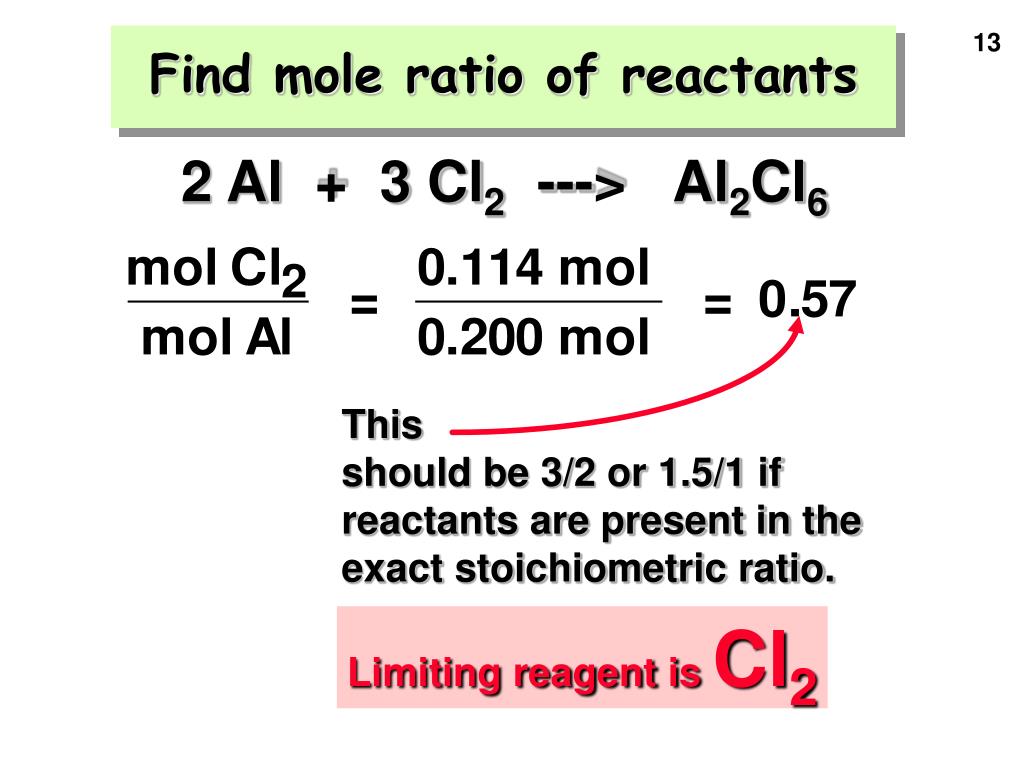
Stoichiometry provides the mathematical framework to pinpoint limiting reactants by comparing mole ratios derived from balanced equations. For any unbalanced equation, converting masses or volumes of reactants into moles enables direct comparison of their relative amounts.The reactant whose stoichiometric coefficient multiples its measured moles to a value less than that of others defines the limit.
This process hinges on precise molar conversions: mass to moles via molar mass, and volume to moles for dilute solutions using density or concentration. For example, reacting 10 grams of calcium (Ca, molar mass 40 g/mol = 0.25 mol) with 10 grams of hydrochloric acid (HCl, molar mass 36.5 g/mol ≈ 0.274 mol) requires analyzing 1:2 Ca:2HCl ratio.
With 0.25 mol Ca and 0.274 mol HCl, HCl is in excess; Ca governs product formation.
Step-by-Step Identification of Limiting Reactants
1. Write or verify a balanced chemical equation, ensuring correct coefficients. 2.Convert masses or volumes of reactants to moles using molar masses or concentration formulas. 3. Calculate how many moles each reactant represents.
4. Use stoichiometric ratios from the equation to determine the maximum product possible per reactant. 5.
Compare resulting amounts—smaller is the limiting reactant, largest the excess.
This systematic approach minimizes errors in lab reports and production processes. A lab technician scaling a synthesis from grams to moles must apply this method rigorously to avoid shortfall or overuse.
In industrial settings, even minor miscalculations can mean millions wasted or safety hazards compounded.
Critical Implications in Manufacturing and Research
Limiting reactant theory directly influences process optimization. In manufacturing, maximizing conversion to product while minimizing unreacted materials reduces waste and cost. For bulk chemical production—such as ammonia synthesis via the Haber process—accurate limiting reactant analysis prevents incomplete conversion and exhausts raw materials’ economic potential.Beyond quantity, the principle safeguards reaction safety. Reactions fuelled beyond exposure to limiting reactants may proceed exothermically past control, increasing risks in reactors. In pharmaceutical synthesis, where purity and yield define success, limiting reactant determination ensures precise stoichiometric trips to active pharmaceutical ingredients, reducing impurities from excess or incomplete reactions.
Classroom Applications and Educational Significance
In academic settings, limiting reactants anchor foundational stoichiometry lessons.Students learn not just equations but real-world relevance—balancing equations, using molarity, and solving multi-reactant problems cultivate analytical thinking. Inquiry-based labs reinforce concepts by challenging learners to predict and verify yield limitations. This hands-on engagement transforms abstract math into tangible scientific insight, preparing future chemists for challenges in research and industry.
Demonstrations using titrations or fixed-volume gas reactions vividly show how one reactant’s depletion halts progress, embedding deep conceptual mastery. Such experiences underscore the necessity of limiting reactants long after classroom walls close.
The Unseen Engineer Behind Chemical Efficiency
Though rarely visible, the limiting reactant acts as a silent engineer shaping chemical processes. In every reaction, from a test tube to a factory pipeline, this principle governs precision and outcome.Its careful determination ensures resource efficiency, product purity, and operational safety. Mastery of limiting reactants elevates scientific practice from guesswork to deliberate control—turning chemical equations from symbols into executable, efficient, and scalable workflows.
In essence, the limiting reactant is not merely a theoretical concept but a cornerstone of practical chemistry, safeguarding innovation across science and industry.
:max_bytes(150000):strip_icc()/simple-experiment-58b5b3325f9b586046bbfa7f.jpg)

Related Post

Emma Auturin Pioneers Emotional Resilience: The Neuroscience Behind a Visionary Life
WWE Showing Signs Of Even More NFT Action

Megan Telles Ktla: A Profile of Resilience, Family, and Identity
Unlocking the Secrets of Ni Molar Mass: The Essential Metric That Drives Chemistry

