The Chemical Backbone of Life: Decoding the Essential Elements of Nucleic Acids
The Chemical Backbone of Life: Decoding the Essential Elements of Nucleic Acids
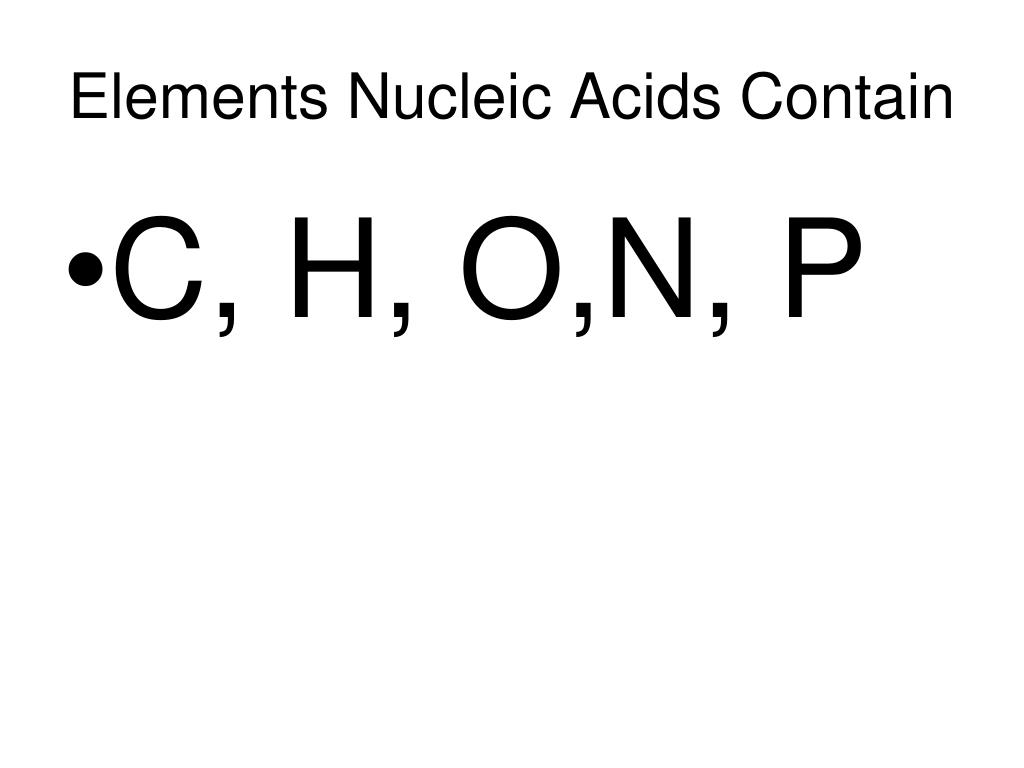
Nucleic acids—the molecular architects of heredity and vital regulators of cellular function—derive their extraordinary biological power from a precise arrangement of chemical elements. At the core of DNA and RNA lie five fundamental building blocks: oxygen, carbon, hydrogen, nitrogen, and phosphorus. These elements form the structural framework and functional centers of nucleic acids, enabling the storage, transmission, and expression of genetic information.
Understanding the chemical roles of these elements unlocks deeper insight into how life’s blueprint is encoded at the atomic level.
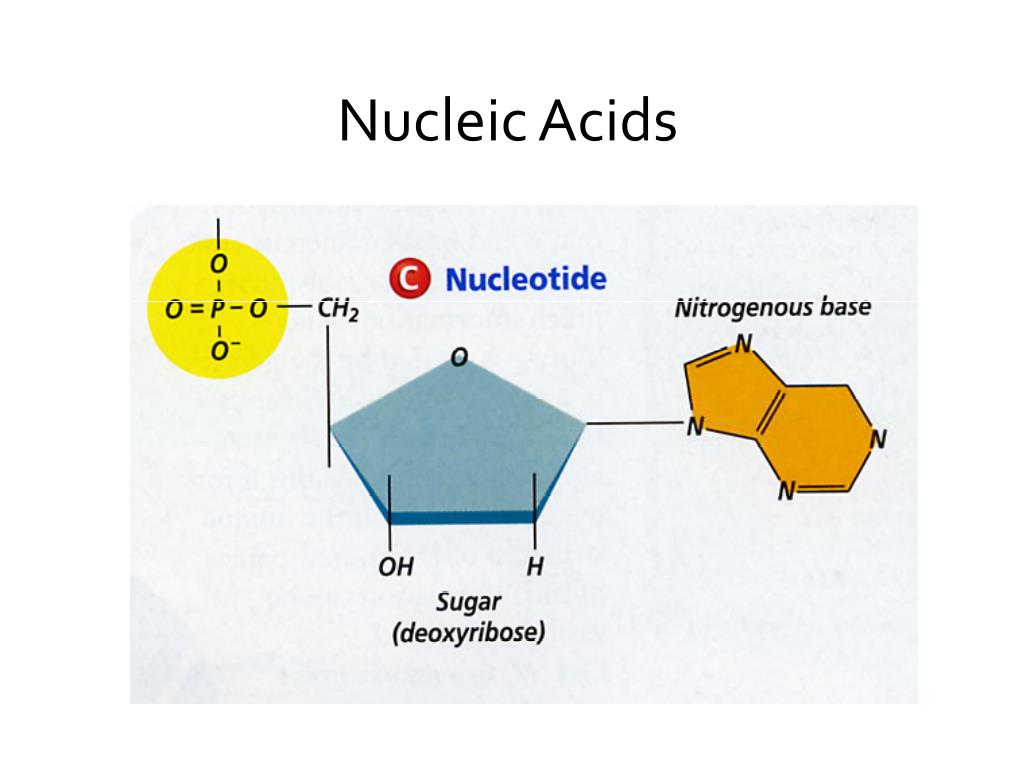
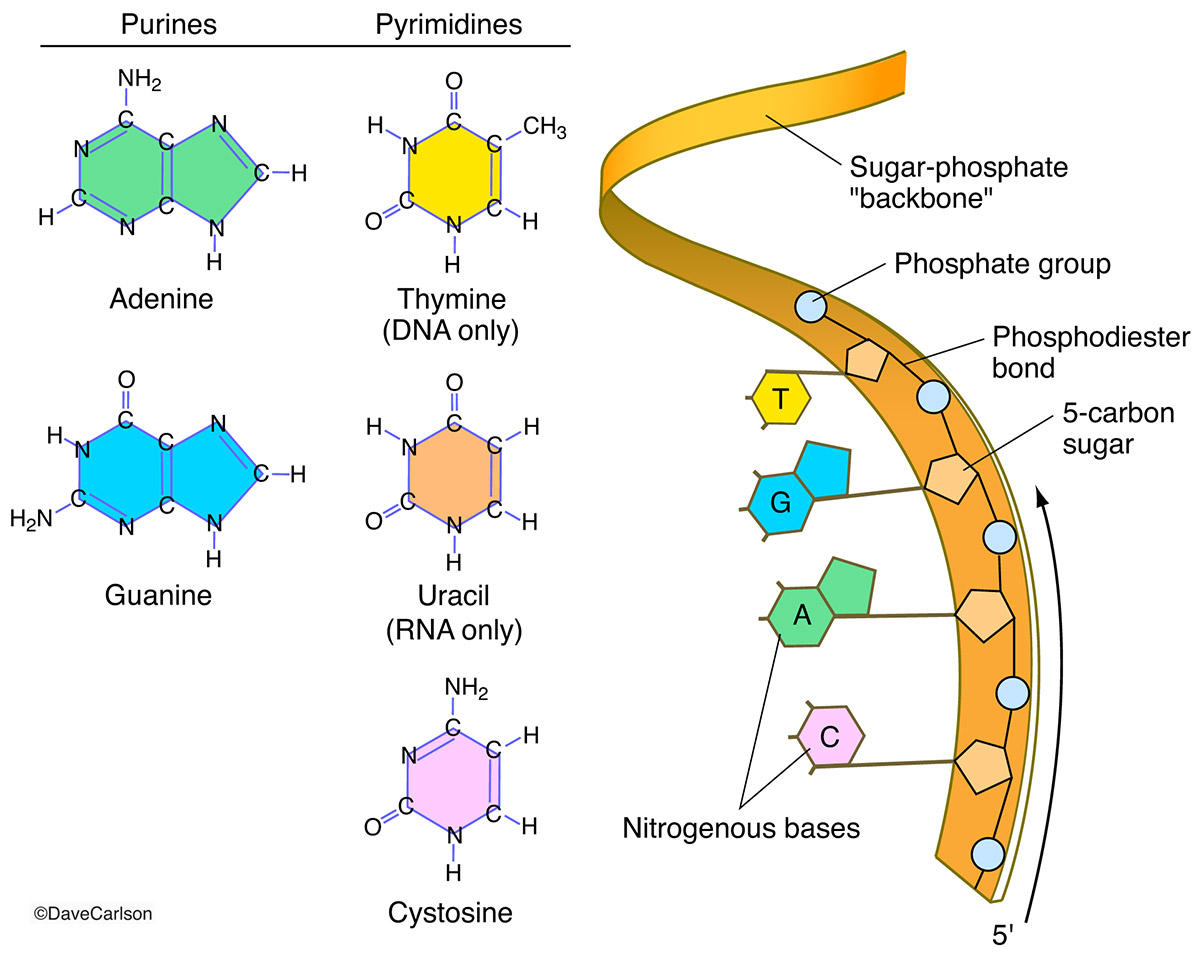
Each nucleic acid—DNA (deoxyribonucleic acid) and RNA (ribonucleic acid)—is composed of nucleotide monomers, each featuring a nitrogenous base, a pentose sugar, and a phosphate group. The significance of phosphorus cannot be overstated: it acts as the molecular tether, linking sugar units through phosphodiester bonds that form the backbone.
Without phosphorus, the linear, stable chain of nucleic acids could not exist. As biochemist David L. Sforza notes, “Phosphorus is the silent architect, anchoring nucleic acid strands with extraordinary strength and directionality.”
The Foundation: Carbon and Hydrogen in Sugar Scaffolds
The sugar component—either deoxyribose in DNA or ribose in RNA—is built primarily from carbon and hydrogen atoms arranged in a five-membered ring.This deoxyribose (C₅H₁₀O₅) and ribose (C₅H₁₀O₅) framework provides both structural rigidity and chemical versatility. The specific placement of carbon atoms determines stereochemistry—such as the absence of a 2’ hydroxyl group in DNA’s deoxyribose versus the 2’ OH in ribose’s sugar—critical for DNA’s stability and RNA’s reactivity. Hydrogen bonds between sugar and phosphate groups further stabilize the biological architecture.
These transient but crucial forces govern folding, replication, and repair, making carbon-hydrogen chemistry essential not just for structure, but for dynamic biological function.
The sugar-phosphate backbone, though chemically straightforward, enables complex behaviors. Its ability to carry a negative charge due to phosphate groups attracts cations like magnesium and sodium, which stabilize nucleic acid conformation in cellular environments.
As molecular biologist Jennifer Doudna emphasized, “The elegance of nucleic acids lies in how a few elements yield infinite complexity through precise atomic interactions.”
Nitrogen: The Genetic Code’s Alphabet
Nitrogen atoms form the essential heterocyclic bases—adenine, guanine, cytosine, thymine (in DNA), and uracil (in RNA)—that encode genetic information. These nitrogen-containing rings—the purines (adenine and guanine) and pyrimidines (cytosine, guanine, thymine, uracil)—create a four-letter language toward which biological meaning is built. The arrangement and pairing of these bases drive DNA replication and RNA transcription through hydrogen bonding specificity: adenine pairs with thymine or uracil, guanine with cytosine.The nitrogen in these bases also influences molecular stability and reactivity. Their electron-rich structures permit selective base pairing, while subtle variations in molecular geometry ensure fidelity during genetic processes. As discovered through X-ray crystallography and spectroscopy, even minor changes—such as the removal of a 2’ hydroxyl in DNA’s ribose—profoundly affect base-pairing accuracy and chemical resilience.
Beyond storing genetic code, these nitrogenous bases interface with proteins. For instance, RNA’s nitrogenous sequence governs protein synthesis by directing amino acid assembly via codons—a direct translation of elemental chemistry into life-sustaining action.
Phosphorus: The Key to Genetic Continuity
Phosphorus is indispensable in nucleic acids for two primary reasons: forming phosphodiester linkages and conferring chemical stability. Each phosphate group links adjacent nucleotides in the 5’ to 3’ direction, constructing the directional phosphodiester backbone that resists hydrolytic breakdown and maintains genetic integrity.This linkage, uniquely governed by phosphorus, enables DNA and RNA to persist over generations and mediate transient but vital functions. Phosphorus also contributes phosphate groups to energy-carrying molecules like ATP, indirectly sustaining the enzymatic processes that replicate and transcribe nucleic acids. Without phosphorus, nucleic acids would collapse into unstructured polymers, rendering heredity and gene expression impossible.
The Elemental Symphony in Molecular Function
Beyond structural roles, the elemental composition of nucleic acids enables nuanced biological regulation. Phosphorus-backed backbones allow DNA to refract X-rays—a discovery pivotal to elucidating its double helix structure. The charge imbalance created by phosphate groups facilitates interactions with histones and enzymes, orchestrating chromatin organization and repair.Similarly, nitrogen’s base-pairing specificity ensures accurate genetic replication, while the slight acidity of phosphate groups enables buffering capacity critical to cellular pH homeostasis. Each element contributes to a coordinated system where chemistry becomes biology. The interplay of carbon-hydrogen stability, nitrogen’s genetic alphabet, and phosphorus’s structural and energetic roles forms a molecular symphony that underpins life itself.
Modern techniques such as cryo-electron microscopy and mass spectrom

Related Post
Unveiling The Secrets Of Ippa 010054: A Comprehensive Guide To Its Significance And Application

Sunspired Drives Solar Innovation: How Cutting-Edge Design Powers Next-Gen Energy Solutions
Nadia Caterina Munno Pasta Queen Age

48Kilos to Pounds: The Crucial Conversion Every Traveler, Athlete, and Health Enthusiast Must Know

