The Atomic Key to Life: How Oxygen’s Proton Count Defines the Element
The Atomic Key to Life: How Oxygen’s Proton Count Defines the Element
Oxygen, the eighth element on the periodic table and a vital breath for nearly all known life, holds a fundamental property that shapes its chemistry, behavior, and central role in biology: it contains eight protons. This single numerical truth underpins oxygen’s electron structure, reactivity, and the molecule it forms—O₂—a diatomic gas essential to respiration and combustion. With precisely eight protons in each atom, oxygen occupies a unique position in the building blocks of matter, influencing everything from cellular metabolism to planetary atmospheres.
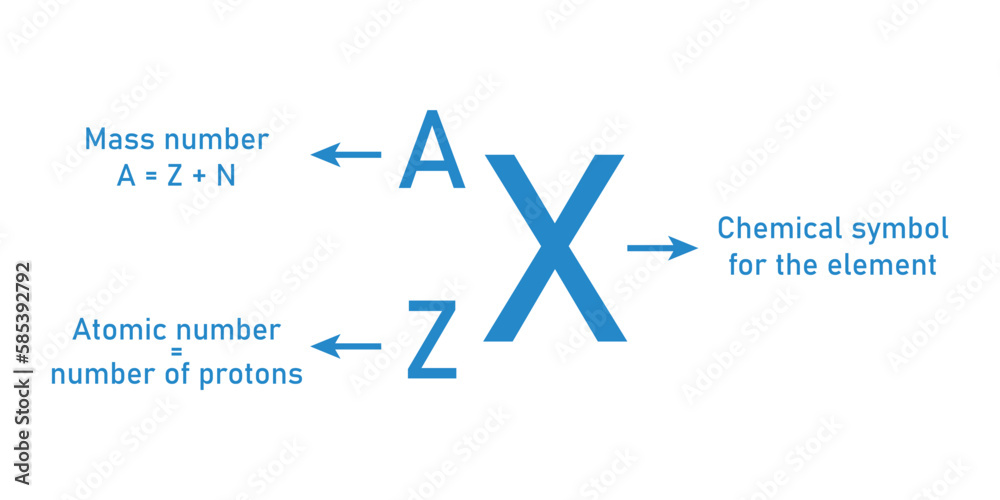
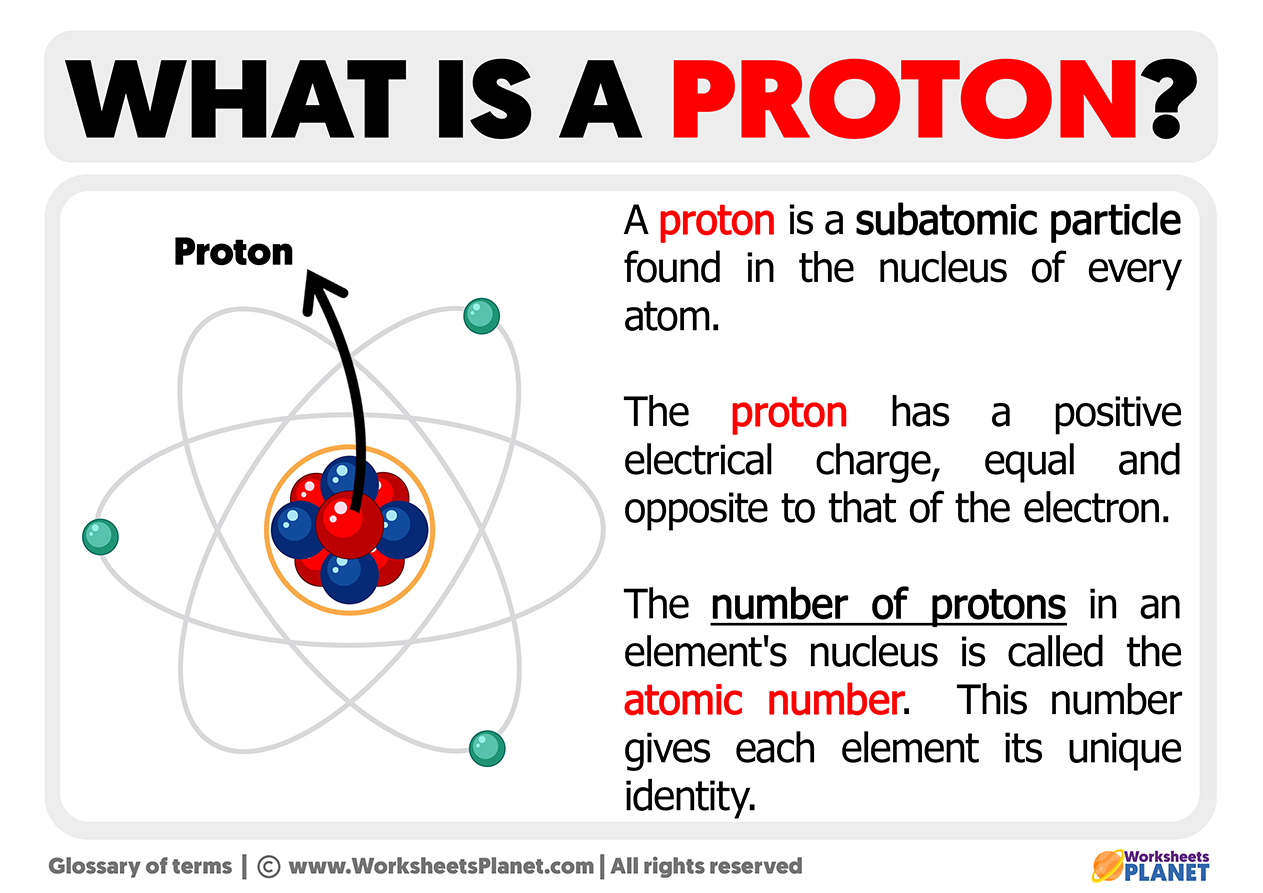
Each oxygen atom’s atomic number of 8 defines its entire identity. Protons, positively charged particles in the atomic nucleus, determine the element’s identity and cumulative electrical character. Oxygen’s eight protons give rise to an electron configuration of 1s² 2s² 2p⁴, which governs how it bonds, interacts, and sustains life.
Understanding this proton count is not merely academic—it is foundational to grasping oxygen’s indispensable place in chemistry, biology, and environmental science. The architecture of oxygen’s atomic nucleus — eight protons packed into a dense core — sets the stage for its chemical behavior. These protons generate a positive charge strong enough to attract electrons efficiently, yet balanced by the electron shell’s configuration.
The 2p⁴ pairing allows oxygen atoms to form two shared pairs in covalent bonds, commonly seen in O₂, enabling stable molecular interactions. As chemist Dr. Elena Vasquez of the Institute for Atomic Science states, “Oxygen’s proton count isn’t just a number—it’s the silent architect of how the element holds together electronically and chemically.” This precise proton count also influences oxygen’s physical properties.
Positioned in Group 16 (the chalcogens) of the periodic table, oxygen exhibits high electronegativity and a strong tendency to gain electrons, driving its pivotal role in redox reactions. The diatomic O₂ molecule, with its double covalent bond and paramagnetic nature due to unpaired electrons, owes its stability and reactivity directly to eight protons locked in a tightly bound nucleus. This structural integrity enables oxygen to participate in cellular respiration, where it powers energy production by accepting electrons in metabolic pathways.
Beyond biology, the number of protons in oxygen atoms has profound implications in science and technology. In nuclear physics, oxygen-16—featuring eight protons and eight neutrons—serves as a common, stable isotope used in medical imaging and research. Its predictable nuclear structure simplifies studies in particle physics.
Meanwhile, in environmental monitoring, tracking oxygen isotopes helps scientists trace water cycles and climate change, with proton variations offering clues about natural processes.
Oxygen’s simplest fact—a single atomic number of 8—unlocks a cascade of scientific insights. From forming breathable air to enabling energy transfer at the molecular level, each proton defines its capacity to bond, react, and sustain life.
The count of protons in oxygen atoms is far more than a statistic—it is the cornerstone of atomic identity and chemical possibility.
The Proton-Driven Architecture of Oxygen’s Electron Structure
At the heart of oxygen’s chemistry lies its electron configuration, directly shaped by eight protons in the nucleus. With electron shells filled according to quantum rules, the first two electrons occupy the 1s orbital, filling it completely. The next two settle in the 2s orbital, while the final four reside in the 2p subshell—specifically, in the 2pₓ, 2pᵧ, and 2p_z orbitals.This 1s² 2s² 2p⁴ configuration means oxygen has six valence electrons, enabling it to share electrons in covalent bonds with remarkable efficiency.
The 2p⁴ electronic structure creates two unpaired electrons in separate orbitals, a configuration that contributes to oxygen’s paramagnetism—a rare trait among diatomic molecules. While most O₂ molecules are diamagnetic due to filled electron pairs, the odd unpaired electrons in the 2p orbitals enhance reactive pathways in biological and chemical systems.
As noted in advanced quantum chemistry texts, “The presence of unpaired electrons in oxygen’s outer shell is

Related Post
Melvin, Megan, and the Furtick Family: How Steven Furtick’s Personal Life Shapes His Ministry Resonance

Delete Voice Packs in Genshin Impact: A Quick Guide

Antonio Inoki: The Ruthless Visionary Who Redefined Wrestling and Inspired a Global Generation