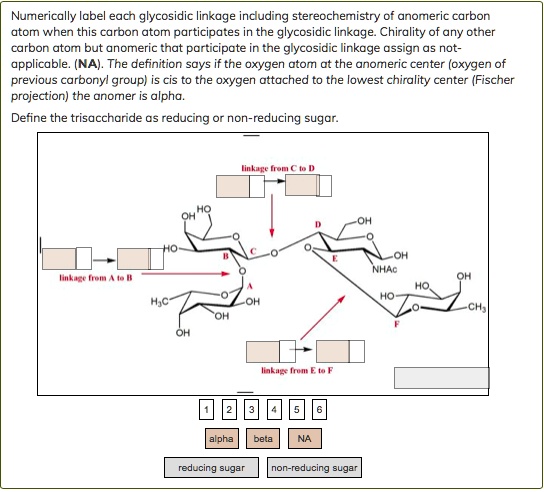
The Anomeric Carbon Atom: Gateway to Sugar Stereochemistry
The Anomeric Carbon Atom: Gateway to Sugar Stereochemistry
The anomeric carbon atom is a pivotal structural element in carbohydrates, serving as the fulcrum around which sugar molecules adopt distinct spatial configurations—critical to their chemical behavior and biological roles. Located at the hemiacetal (or hemiketal, in furanoses) carbon of cyclic sugars, this carbon atom carries the primary site of stereochemical differentiation, enabling the existence of α and β anomers—two optically isomeric forms that differ only in the orientation of the hydroxyl group relative to the ring plane. As expert carbohydrate chemist David K.
N. Mong informs, “The anomeric carbon bridges the flexible ring and the fixed stereochemistry, dictating not just shape but function in enzymatic recognition and molecular assembly.” This atom’s unique position and reactivity underpin the complexity of sugars in biochemistry, from energy storage to molecular signaling.
What Makes the Anomeric Carbon Unique?
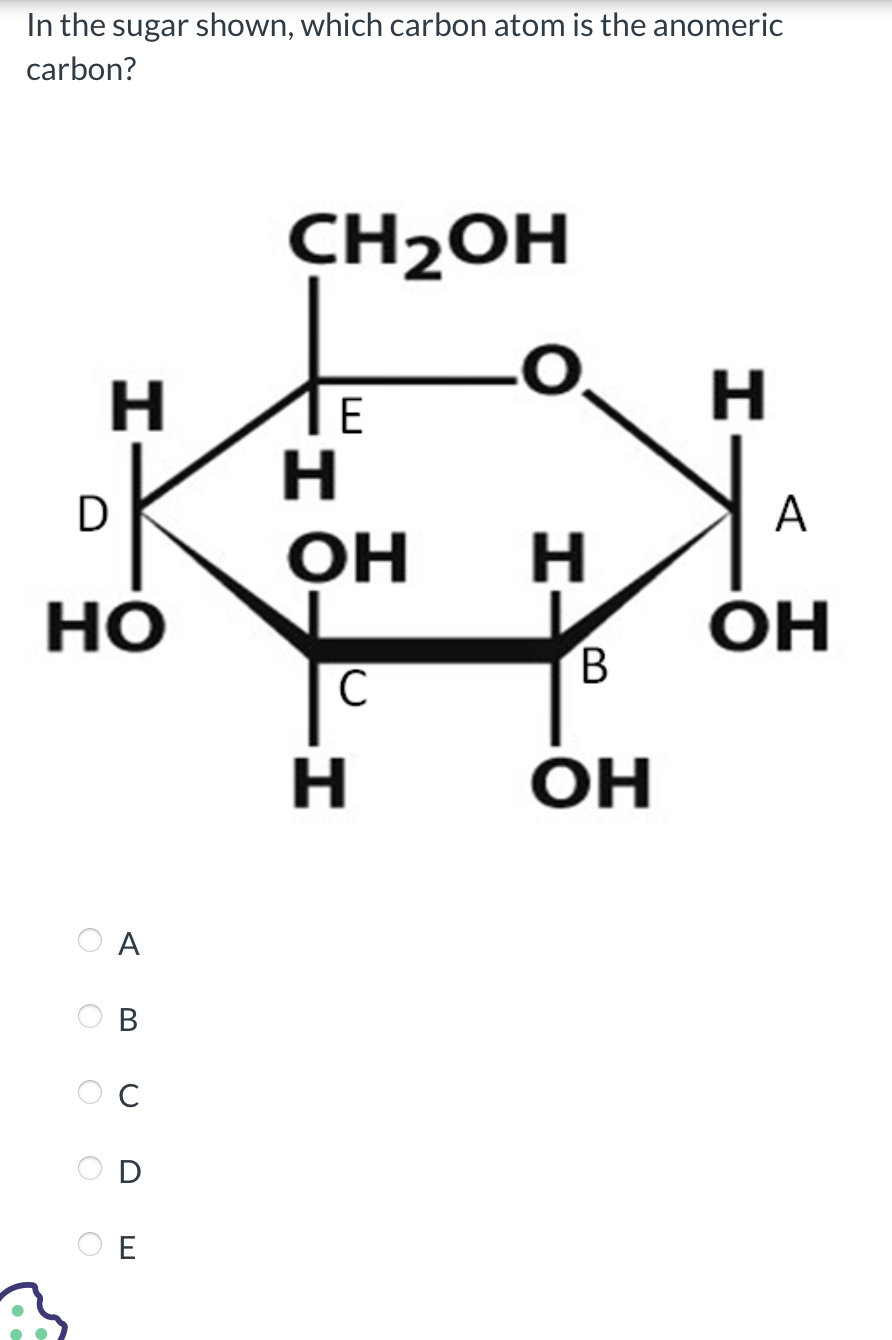
The anomeric carbon, designated as C1 in aldohexoses like glucose or aldopyranoses in furanose forms, differs fundamentally from other carbons due to its dual role in ring formation and reactivity.In cyclic forms such as glucose, the carbonyl carbon (C1) responds to the hydroxyl group’s attack, closing to form either a β-Anderson or α-Anderson half-chair, rotating around the anomeric carbon by ±180°. This rotation generates two stereoisomers: the β form, where the anomeric OH projects below the ring plane, and the α form, with an upward projection. These configurations—termed anomers—are linked by a reversible mutarotation process observed when dissolved in aqueous solution.
This reversible interconversion between α and β forms is central to sugar dynamics, revealed in early 20th-century work by chemists such as Emil Fischer, whose stereochemical models laid the foundation for understanding carbohydrate behavior. In cyclic forms such as pyranose (6-membered ring) and furanose (5-membered ring) sugars, the anomeric carbon (C1 for aldo sugars) becomes the epicenter of structural diversity. The bond formed between C1 and its hydroxyl or substituent dictates whether the terminal hydroxyl projects above (α-anomer) or below (β-anomer), a spatial difference detectable only through polarization or NMR spectroscopy. This subtle change significantly impacts solubility, stability, and interaction with enzymes. For instance, β-D-glucose, more thermodynamically stable than its α counterpart, predominates in natural sources due to its lower energy conformation. The anomeric effect—a stabilizing hyperconjugative interaction between the lone pair on the anomeric oxygen and adjacent π-bonds in the ring—further reinforces β-anomer stability, a phenomenon first identified in benzene derivatives and now central to carbohydrate physics. Biologically, the anomeric carbon is not a passive link but a functional command center. Glycosidic linkages—reactions between the anomeric carbon of one sugar and a hydroxyl group on another—define the architecture of polysaccharides like cellulose, glycogen, and starch, and are essential in glycoproteins and glycolipids vital to cell membranes. Enzymes such as glycosyltransferases precisely recognize and cleave these anomeric bonds, underscoring its role in biochemical specificity. “Without the anomeric carbon,” explains carbohydrate biologist Susan Omura, “life’s sugar-based machinery would lack the foundation for structured communication, energy transfer, and recognition.” This atom thus transitions from a mere chemical node to a dynamic hub of metabolic and structural precision. Isomerization at the anomeric center is not confined to solution. Upon hydrolysis of glycosidic bonds, the anomeric carbon reverts to the open-chain form, revealing a reactive aldehyde or ketone group—chemical hotspots for condensation reactions, metabolic transformations, and analytical techniques like HSPA (High-Performance Sugar Analysis). This reversible state drives glycosidic bond formation, hydrolysis, and enzymatic catalysis, making the anomeric carbon indispensable in both synthetic and natural carbohydrate chemistry. Structural analysis of the anomeric carbon is achievable through NMR spectroscopy, where chemical shifts reflect bond geometry and adjacent functional groups—α-anomers typically exhibit distinct upfield shifts due to electron-donating anomeric OH, while β-anomers show more deshielding. X-ray crystallography further clarifies anomeric stereochemistry at atomic resolution, enabling structural biology to map sugar-protein interactions with near-atomic precision. These tools confirm that even tiny spatial differences at the anomeric carbon can dictate macroscopic properties, from starch gelatinization to viral receptor binding. In synthetic carbohydrate chemistry, manipulating the anomeric carbon is a key strategy. Protective group chemistry—using acetyl or silyl moieties to shield the anomeric OH—allows controlled mutarotation and selective glycosylation. This precision enables the design of novel glycans for drug delivery, vaccine development, and biomimetic materials. The design of therapeutic oligosaccharides, for example, hinges on stabilizing specific anomers to mimic natural signaling motifs or block pathogen entry. The anomeric carbon atom, thus, stands as a microcosm of carbohydrate complexity: small in size, yet immense in consequence. It governs stereochemical identity, influences biochemical interaction, and serves as a nexus between structure and function across biology and chemistry. From fundamental stereochemical principles to cutting-edge therapeutic design, this carbon center remains a cornerstone of sugar science—an irreplaceable pivot in the elegant machinery of life’s molecular choreography.

Related Post
Reyes Connect Your Ultimate Guide: Mastering Integration with Precision and Power
<strong>Master the Best Levels for Diamond Mining: The Ultimate Guide to Minecraft’s Highest Yield Mines</strong>
Tony Khan Was Hoping for Reconciliation with CM Punk Before Recent Social Media Controversy

Exploring The Life And Legacy Of Diahnne Abbott: Voice of Soul and Struggle

