Sulfur’s Valence Electrons: The Molecular Architect Behind Its Chemistry and Reactivity
Sulfur’s Valence Electrons: The Molecular Architect Behind Its Chemistry and Reactivity
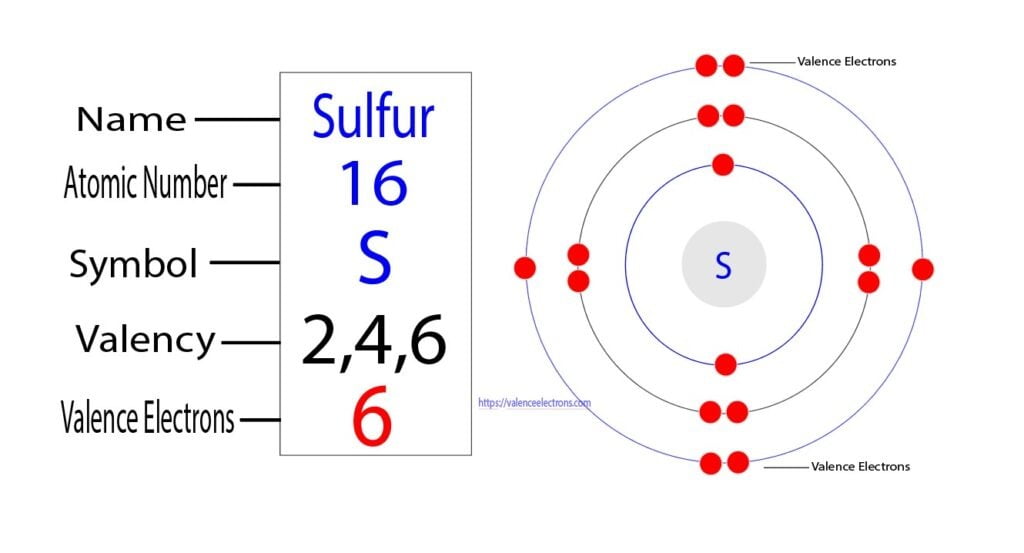
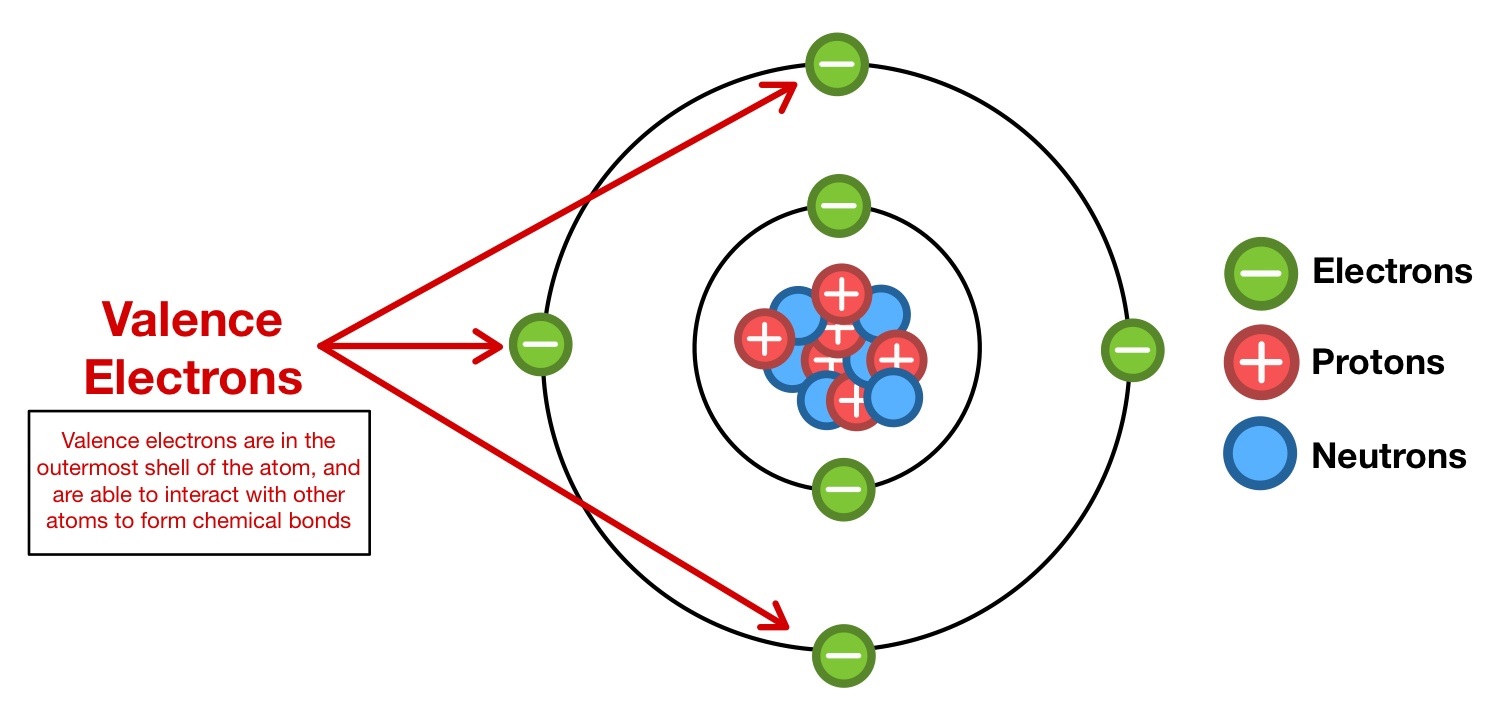
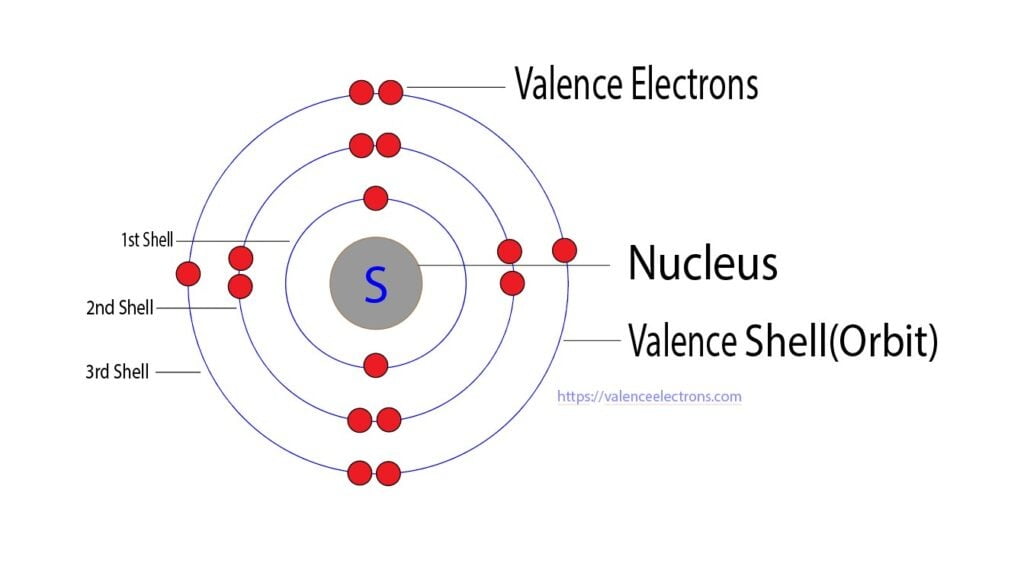
In the intricate world of chemical bonding and molecular behavior, sulfur stands out as a versatile and often underappreciated element—driven fundamentally by its valence electrons. Sulfur, with an atomic number of 16, possesses four valence electrons in its outermost shell, dictating its unique reactivity, bonding patterns, and pivotal role across biological, industrial, and environmental systems. Understanding these valence electrons is key to unlocking sulfur’s chemical personality, from its ability to form diverse oxidation states to its critical participation in key biochemical and geochemical processes.
Sulfur’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁴, placing four electrons in the third energy level. These four valence electrons reside in the 3s and 3p orbitals, conspiring to form a range of chemical bonds. Unlike hydrogen or carbon, sulfur’s multiple accessible outer electrons allow it to engage in octet-bonding scenarios beyond simple duplication—enabling compound formation across a broad range of oxidation states.
This flexibility makes sulfur a linchpin in both organic synthesis and natural biochemical pathways.
Valence Electrons and Bonding Behavior: From Simple to Complex
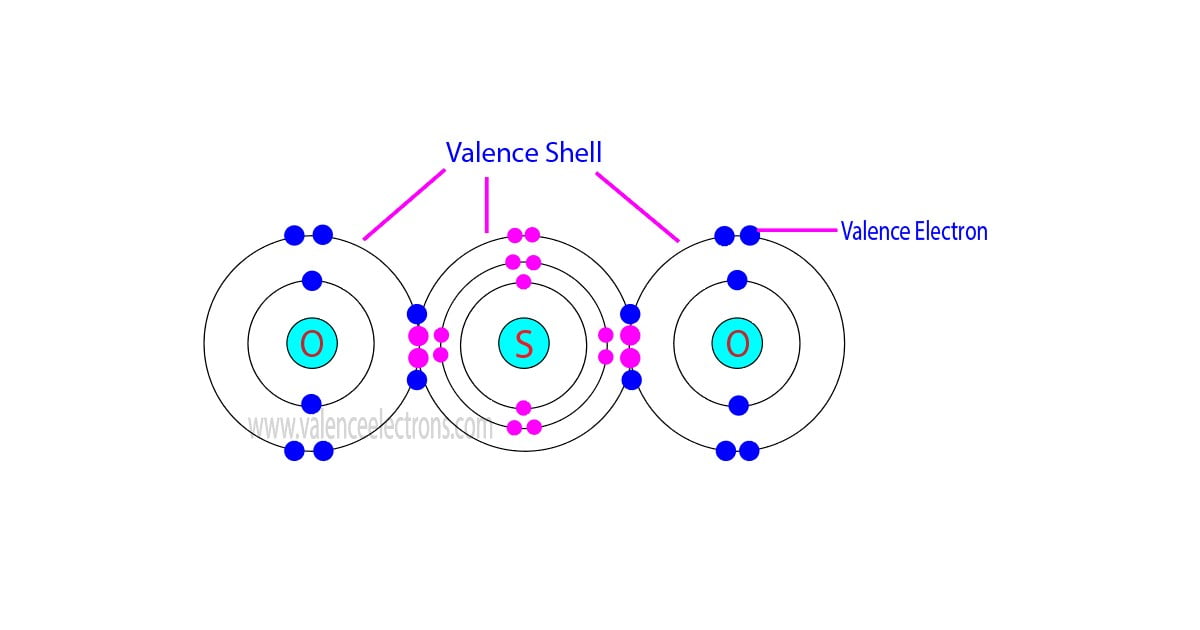
Sulfur’s four valence electrons empower it to form ionic, covalent, and coordinate bonds, each revealing distinct chemical character. In most compounds, sulfur typically uses two of its valence electrons to bond via single covalent bonds—such as in hydrogen sulfide (H₂S), where sulfur shares one electron with each hydrogen, stabilizing its –2 oxidation state. But sulfur’s chemistry expands dramatically when it sheds or shares additional electrons through redox reactions.In sulfate (SO₄²⁻), for example, sulfur achieves an oxidation state of +6 by utilizing all four valence electrons in multiple covalent bonds—four single bonds and shared lone pairs—while remaining stable due to its expanded octet capacity in this high-charge environment.
This capacity to vary oxidation states—from –2 in sulfides to +6 in sulfates—is directly tied to availability of valence electrons for either donation or acceptance. Sulfur’s ability to expand its electron domain is facilitated by vacant d-orbitals, a subtle yet powerful quantum mechanical nuance that allows for sounder electron distribution in higher oxidation states, crucial in mineral and biological sulfur cycles.
The Role in Key Compounds and Industrial Applications
Sulfur’s valence electrons directly influence the structure and stability of its most vital compounds.In elemental sulfur, S₈ rings consist of six sulfur atoms each contributing two valence electrons in a cyclic arrangement stabilized by resonance. This molecular design underpins its use in vulcanization, where sulfur crosslinks rubber polymers—enhancing elasticity and durability. Each sulfur atom’s four valence electrons participate in both intra- and intermolecular interactions, facilitating long-chain network formation.
In industrial contexts, sulfur dioxide (SO₂), formed during incomplete combustion, derives its reactivity from the sulfur’s formal +4 oxidation state—achieved through partial oxidation where valence electrons are redistributed. This molecule remains central to the global sulfur cycle and acid rain formation, yet also serves as a precursor for sulfuric acid (H₂SO₄), the world’s most produced industrial chemical. The sequential oxidation and reduction behaviors hinge on sulfur’s electron mobility, showing how subtle changes in valence state control transformative chemical pathways.
Biological Significance: Sulfur’s Valence Electrons in Life’s Machinery
In biology, sulfur’s four valence electrons are indispensable. Found in critical amino acids like cysteine and methionine, these electrons form sulfide (–SH) groups capable of both covalent disulfide bridges—stabilizing protein tertiary structures—and redox reactions central to cellular metabolism. Disulfide bonds, for instance, form via pairwise electron coexistence between two sulfur atoms, locking protein folds into functional three-dimensional shapes.Quotes from biochemists emphasize this: “Sulfur’s valence electrons allow cysteine to dynamically modulate protein structure and signaling through reversible disulfide exchange—a redox switch crucial in cellular redox homeostasis,” notes Dr. Amara Patel, structural biologist at MIT. Moreover, sulfur’s redox versatility supports enzyme cofactors, electron transport chains, and detoxification processes, underscoring its biological indispensability.
Environmental and Geochemical Impacts
Beyond life and industry, sulfur’s valence electrons shape Earth’s geochemistry. Volcanic emissions release sulfur gases like SO₂, where sulfur assumes variable oxidation states influenced by atmospheric conditions—driven by valence electron dynamics. In sedimentary basins, sulfate-reducing bacteria exploit sulfur’s electron-sharing capacity to convert sulfate into sulfide, releasing hydrogen sulfide (H₂S) with biologically toxic yet geologically significant outcomes.The Cycles That Sustain Planetary Balance
Sulfur’s valence electrons underpin two fundamental biogeochemical cycles: the sulfur cycle and the redox cycle. In the sulfur cycle, atmospheric sulfur (often as SO₂) transforms via microbial redox reactions—oxidation to sulfate (S⁶⁺) and reduction back to H₂S—each phase dependent on electron transfer governed by sulfur’s valence shell flexibility. These transformations regulate nutrient availability, climate feedback, and ecosystem function.The tight linkage between electron availability and environmental transformation underscores sulfur’s planetary importance.
Final Thoughts: Sulfur’s Valence Electrons — The Silent Drivers of Molecular Diversity
Sulfur’s four valence electrons are far more than a quantum detail—they are the foundation of sulfur’s remarkable versatility. From forming high-energy polymers to enabling life-sustaining protein structures and mediating geological transformations, the molecule’s reactivity and adaptability stem from how those electrons orchestrate bonding, oxidation, and redox behavior.In chemistry, biology, and Earth sciences, sulfur stands as a testament to how fundamental atomic properties generate complex, global change. Understanding its valence electrons is not just academic—it is essential to harnessing sulfur’s power safely and sustainably.
Related Post
Gracie Parker’s Onlyfans Leak Ignites Controversy: Industry Insiders Weigh In on Impact, Access, and Reputational Fallout
The Enduring Power and Evolving Landscape of Monopoly Online
The Financial Triumph of a Rap Queen

ABillion How Many Zeros? The Unbelievable Scale of Numerical Magnitude Under the Abillion Threshold

