States of Matter and Phase Changes: The Chemistry Behind Everyday Transformations
States of Matter and Phase Changes: The Chemistry Behind Everyday Transformations
The world around us shifts constantly—ice melting into water, steam condensing into droplets, and glaciers flowing over centuries. These visible transformations are governed by fundamental chemical principles: the states of matter and phase changes. Governed by temperature and energy, these processes reveal the dynamic interplay between molecular motion and external conditions.
From the evaporation of water to the solidification of metals, every stage reflects precise scientific rules that shape both nature and human innovation. Understanding states of matter and phase changes is essential not only for high school chemistry learners but for anyone seeking to grasp the invisible forces shaping daily experience.
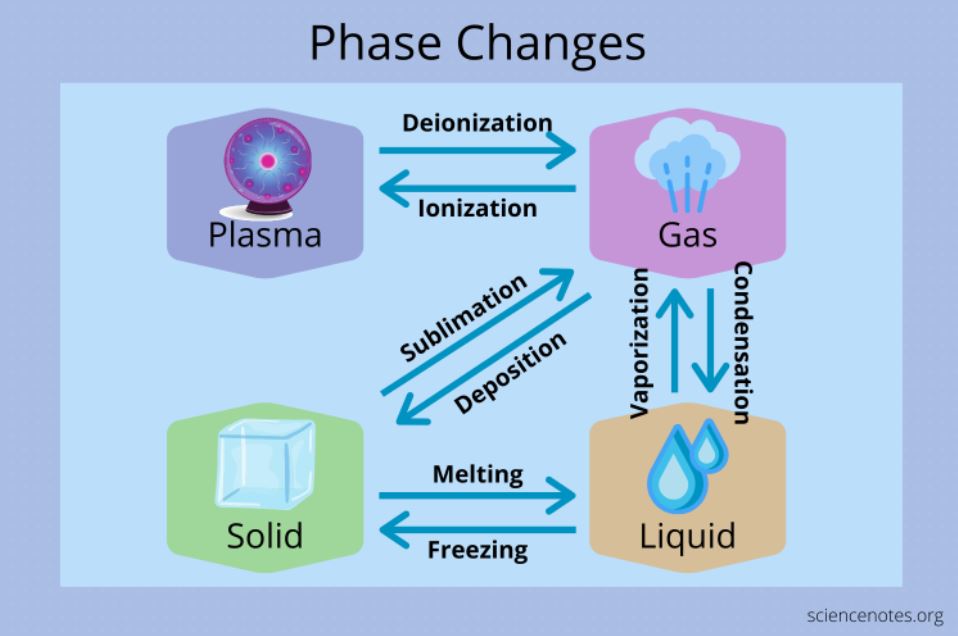
States of matter classification—solid, liquid, gas, and plasma—depends on molecular arrangement and kinetic energy.
Solids exhibit tightly packed molecules with minimal movement; their shape and volume remain fixed. Liquids, while still bound, allow molecules to slide past one another, permitting flow yet maintaining volume. Gases feature widely separated molecules moving freely, filling containers completely.
The rare plasma state, ionized at extreme temperatures, exists in stars and lightning. “Phase is defined by a substance’s physical state, determined by temperature and pressure,” explains physical chemistry principles long taught in high school curricula. “It is not simply a label—it is a measurable condition where molecular interactions dictate behavior.”
Phase changes occur when energies shift, rearranging molecular forces.
The three most common transitions—melting, vaporization, and freezing—highlight energy’s role. When ice absorbs heat, its rigid structure breaks: water molecules gain kinetic energy and overcome fixed hydrogen bonds, transitioning from solid to liquid in a process known as melting. This absorption of heat without temperature rise—until all ice melts—is endothermic.
“Melting is the point where solid and liquid phases coexist, with energy used to break intermolecular bonds,” notes a standard high school chemistry reference.
Similarly, vaporization involves transforming liquid into gas, requiring energy to overcome attractive forces between molecules. Evaporation, the surface-layer vaporization at ambient temperatures, differs from boiling, where vapor forms throughout a liquid upon reaching its boiling point.
“Boiling occurs when vapor pressure equals external pressure,” explains the Concise High School Chemistry PDF, underscoring the thermodynamic balance governing phase behavior. Condensation—gas reverting to liquid—releases energy, visible in morning dew or steam dissipating in a cooling cup of tea.
Freezing and melting, inversely related, mark reversible transitions essential to climate and biology.
Water’s unique position—expanding upon freezing—stems from hydrogen bonding creating open ice structures. This anomaly protects Earth’s ecosystems, as floating ice insulates liquid water below. “Water’s phase behavior is special,” reiterated in multiple educational guides, “and underpins countless natural phenomena.” Sublimation, where solid transitions directly to gas without melting—observed in dry ice—further illustrates phase variability under low-pressure conditions.
Phase diagrams serve as blueprints to predict molecular behavior under varying temperatures and pressures. These graphical representations plot melting and boiling points, triple points (where solid, liquid, gas coexist), and critical points (beyond which gas and liquid vanish). At the triple point, for example, water’s solid, liquid, and gas phases coexist in equilibrium.
“Understanding phase diagrams allows scientists and engineers to design processes ranging from cryogenics to industrial distillation,” the PDF emphasizes. Students routinely analyze these graphs to interpret phase boundaries crucial to chemistry’s practical applications.
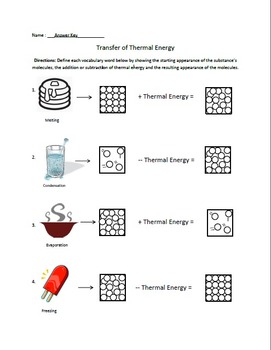
Latent heat—the energy absorbed or released during phase change without temperature change—further reveals hidden thermal dynamics.
Latent heat of fusion and vaporization quantify these energy exchanges: 334 joules per gram for ice melting, and 2260 k