Sn1 vs. Sn2: The Chemical Battle That Defines Reaction Engineering
Sn1 vs. Sn2: The Chemical Battle That Defines Reaction Engineering
In the high-stakes world of organic chemistry, two fundamental reactions—SN1 and SN2—dictate the fate of countless molecular transformations. While both mechanisms enable nucleophilic substitution, they operate through dramatically different pathways, speed profiles, and stereochemical outcomes. Understanding the nuances between them is essential not only for academics but for industrial chemists, pharmaceutical developers, and materials scientists who rely on precise control over reaction design.
The tension between SN1’s stepwise, carbocation-driven kinetics and SN2’s concerted, backside attack profile shapes modern synthetic strategies, often determining whether a reaction succeeds or fails on the lab bench.
Core Mechanisms: A Tale of Two Pathways
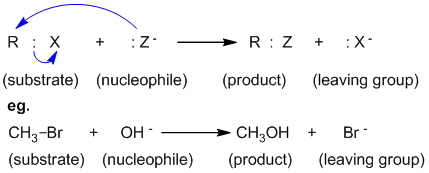
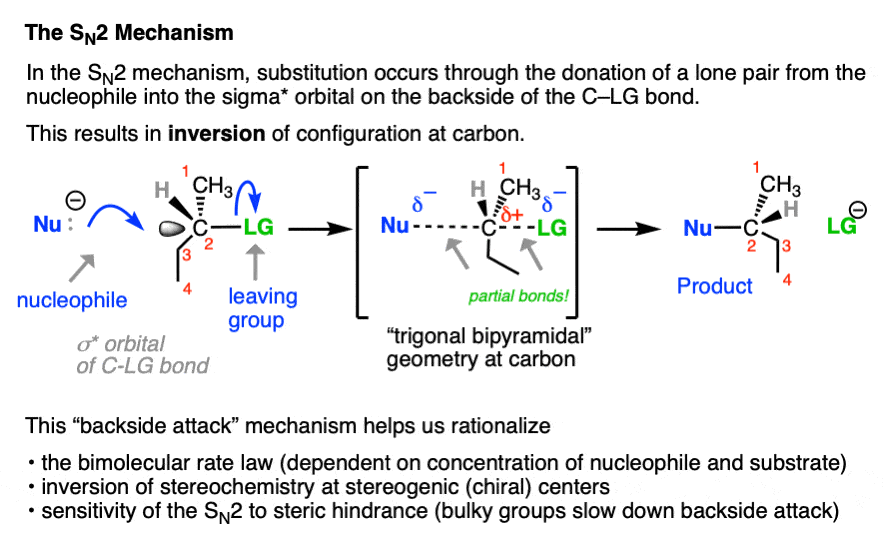
SN1 (Substitution Nucleophilic Unimolecular) reactions unfold in a two-step cascade, beginning with the slow, rate-determining dissociation of a leaving group to generate a reactive carbocation intermediate. This step defines the reaction’s dependence solely on the concentration of the substrate: rate = k[substrate]. “Carbocation stability becomes the key player,” notes organic chemistry professor Dr.Elena Torres. “Tertiary substrates dominate here because they form stable, res contributory intermediates—something primary substrates simply cannot do.” In contrast, SN2 (Substitution Nucleophilic Bimolecular) proceeds in a single, synchronized step: the nucleophile attacks the electrophilic carbon from the opposite side of the leaving group, causing a simultaneous bond break and bond formation. This backside attack triggers an inversion of configuration—earning SN2 its signature stereochemical signature—while the rate hinges equally on both substrate and nucleophile concentration: rate = k[substrate][nucleophile].
“It’s a race at the molecular level,” explains Dr. Rajiv Mehta, synthetic chemist at MIT. “No lingering carbocations—just precision in one unified moment.”
Kinetic Differences: Speed and Dependence
Kinetics lie at the heart of distinguishing SN1 and SN2.SN1 reactions exhibit first-order kinetics, meaning the rate depends exclusively on substrate concentration. A doubling of substrate mass doubles the reaction speed—a hallmark of unimolecular processes. By contrast, SN2 reactions are second-order, sensitive to both substrate and nucleophile concentration, reflecting their concerted nature where multiple events occur simultaneously.
This distinction has profound practical implications. In a lab focused on rapid screening, an SN1 reaction might be favored when substrate stability ensures consistent yield, even at low nucleophile concentration. Conversely, SN2’s sensitivity to both components allows fine-tuned control, especially in polar aprotic solvents that enhance nucleophilicity.
“It’s not just about speed; it’s about predictability,” says chemical engineer Naomi Lin. “SN1 offers robustness in crowded reaction environments, while SN2 delivers precision—ideal for complex molecule synthesis where stereochemistry matters.”
Stereochemistry: Inversion, Retention, and Identity
The fate of configuration in substitution reactions reveals one of SN1 and SN2’s most dramatic differences. SN2 reactions always invert the stereochemistry of the chiral center, a transformation dictated by electrostatic repulsion and steric constraints during the backside attack.If the substrate is (R), the product will be (S)—a rule so consistent it’s foundational in stereochemical planning. SN1 reactions, by enabling carbocation intermediates, erase stereochemical memory. The planar, sp²-hybridized carbocation intermediate welcomes nucleophilic attack from either face, leading to a racemic mixture when the starting material is chiral.
This racemization can be a liability in pharmaceutical development, where stereochemistry directly influences drug efficacy and safety. “Some medicines require only one enantiomer,” cautions Dr. Torres.
“An SN1 path risks producing both forms—undesirable and perhaps dangerous.”
Substrate Preferences: Structural Determinants
Substrate structure is the linchpin governing SN1 or SN2 dominance. Tertiary alkyl halides nearly exclusively favor SN1 due to hyperconjugation and steric protection of the carbocation. Secondary substrates can favor either pathway depending on stability, but generally lean toward SN1 in protic solvents.Primary substrates, lacking sufficient carbocation stability, almost never undergo SN1; instead, they demand the bimolecular precision of SN2. Solvent choice further amplifies these preferences. Protic solvents—like water or alcohols—stabilize carbocations through hydrogen bonding, perfectly complementing SN1’s stepwise mechanism.
Polar aprotic solvents—such as DMSO or acetone—rehydrate nucleophiles, boosting their reactivity in SN2 reactions without stabilizing developing charges in the transition state. Mixed or alternative solvents offer adjustment, but the substrate’s inherent stability underlies the outcome. Adding nucleophiles into this equation reveals another layer: SN2 favors strong, hard nucleophiles (e.g., hydroxide, alkoxides) capable of direct attack, while SN1 reactions require only a good leaving group—strong electrophiles like sulfates or tosylates—regardless of nucleophile strength.
“Understand the dance between substrate integrity and reagent nature,” advises chemical technologist Marco Alvarez. “It’s not just chemistry—it’s about engineering the environment for success.”
Real-World Applications: From Pharmaceuticals to Polymers
The practical impact of SN1 versus SN2 transcends theory. In pharmaceutical synthesis, chemists exploit SN2 to selectively invert stereochemistry in chiral drug precursors, ensuring the active enantiomer is delivered cleanly.Meanwhile, SN1 dominates in industrial processes requiring stable, high-yielding transformations—such as the production of tert-butyl derivatives, where carbocation intermediates withstand harsh conditions. In agrochemicals, SN2 reactions enable rapid functionalization of heterocyclic nuclei, accelerating the development of next-generation herbicides and insecticides. Even in polymer science, SN2-like mechanisms underpin the efficient formation of polymer chains via nucleophilic ring-op


Related Post

SN1 vs SN2: The Chemical Dance of Substitution Reactions

Unlocking Nigel Nelson's Legacy: Unveiling Groundbreaking Discoveries That Redefined Science

