Revealing Aromatic Secrets: How Ir Spectroscopy Deciphers the Irrous Allure of the Aromatic Ring
Revealing Aromatic Secrets: How Ir Spectroscopy Deciphers the Irrous Allure of the Aromatic Ring
When the electron cloud of a benzene-like structure quietly vibrates at specific frequencies under infrared light, a hidden world of molecular identity emerges—this is where Ir spectroscopy becomes the detective of aromatic rings. The core of organic chemistry’s structural language lies in aromaticity, and infrared spectroscopy serves as a precise tool to decode these stable, ring-shaped conjugated systems. By analyzing the distinct vibrational signatures of carbon-hydrogen bonds within aromatic systems, IR spectroscopy reveals not only presence but also environment, substitution, and even aromaticity strength.
Understanding how functional groups interact with the π-electron system within the ring enables researchers to identify compounds ranging from pharmaceutical intermediates to advanced polymers. Ir spectroscopy leverages the unique ability of aromatic rings to absorb infrared radiation at characteristic frequencies, primarily arising from C–H stretching, C=C bending, and C–aromatic C–H resonances. “The aromatic ring’s fingerprint lies in its low-energy stretching modes and subtle bend deformations,” explains analytical chemist Dr.
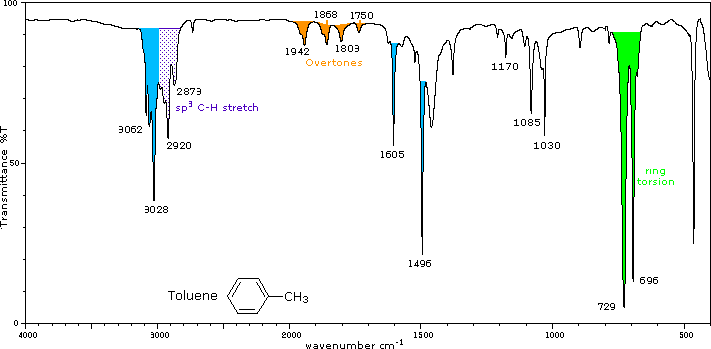
Elena Markovitch. “These vibrations reflect the delocalized π-electron system—distinct from aliphatic compounds, giving clear diagnostic peaks.” For example, the in-plane C–H stretch of aromatic systems typically falls between 3000–3100 cm⁻¹, while C–H bending modes appear near 600–800 cm⁻¹—frequencies markedly different from saturated C–H stretches above 3000 cm⁻¹. The power of IR spectroscopy lies in its sensitivity to subtle structural variations.
Ring substitution—be it electron-donating or electron-withdrawing groups—alters vibrational frequencies in predictable ways. Methyl groups on benzene, for instance, produce a strong C–H stretch near 3020 cm⁻¹, slightly shifted compared to vanilla derivatives with electron-withdrawing substituents, which may dampen or shift the signal. This responsiveness allows scientists to not only confirm aromaticity but also deduce substitution patterns and even molecular conformation.
Key absorptions defining aromatic rings in IR spectra include:
3020–3100 cm⁻¹ – Aromatic C–H stretching modes, sharp and robust, indicative of conjugated ring systems.
600–800 cm⁻¹ – C–C out-of-plane bending vibrations, strong evidence of planar ring geometry and aromatic stability.
1600–1650 cm⁻¹ – Aromatic C=C stretching region, a critical benchmark—though less diagnostic alone, shifts correlate with ring substitution and electronic effects.
These spectral markers provide a molecular barcode. Unlike NMR, IR requires minimal sample preparation and delivers rapid identification, making it indispensable in quality control, forensic analysis, and synthetic monitoring. In pharmaceuticals, verifying aromaticity via IR confirms target compound integrity early in drug development, reducing costly errors.For materials science, tracking aromatic ring vibrations aids in optimizing conductive polymers where π-conjugation dictates electronic performance.
However, interpreting aromatic signals demands attention to nuance. Overlapping vibrations from functional groups—such as aldehydes or nitro groups near similar frequencies—can obscure ring fingerprints.
Attribute analysis and complementary techniques like Raman spectroscopy or NMR help resolve ambiguities, ensuring accurate assignment. “No single IR peak tells the whole story,” cautions Dr. Markovitch.
“It’s the pattern, context, and corroboration with other data that deliver definitive conclusions.”
This precision makes Ir spectroscopy more than a screening tool—it’s a cornerstone in the analytical toolkit deciphering aromatic rings. Its role extends beyond identification into understanding reactivity, intermolecular interactions, and kinetic stability influenced by substitution patterns. For researchers navigating the complex landscape of conjugated systems, Ir spectroscopy remains a reliable, high-impact method that translates molecular vibrations into actionable chemical insight.In essence, the aromatic ring speaks in vibrations, and Ir spectroscopy listens with keen, revealing clarity. From detection to detailed structural diagnosis, it bridges the gap between abstract molecular concepts and tangible chemical understanding—proving that behind every aromatic bond lies a story written in the language of infrared light.



Related Post

The Commodore 64: Britain’s Amiga-clone That Defined a Generation

Dr Morrow Organism VRchat: A Gateway to Alien Biology Like Never Before
Ashley Iaconetti Podcast Bio Wiki Age Wedding Bachelor Season Salary And Net Worth

Fired Fox Anchors: The Iron Voices Redefining Television Authority

