Pyridine: Your Guide To This Essential Organic Compound
Pyridine: Your Guide to This Essential Organic Compound
The Silent Cornerstone of Modern Chemistry
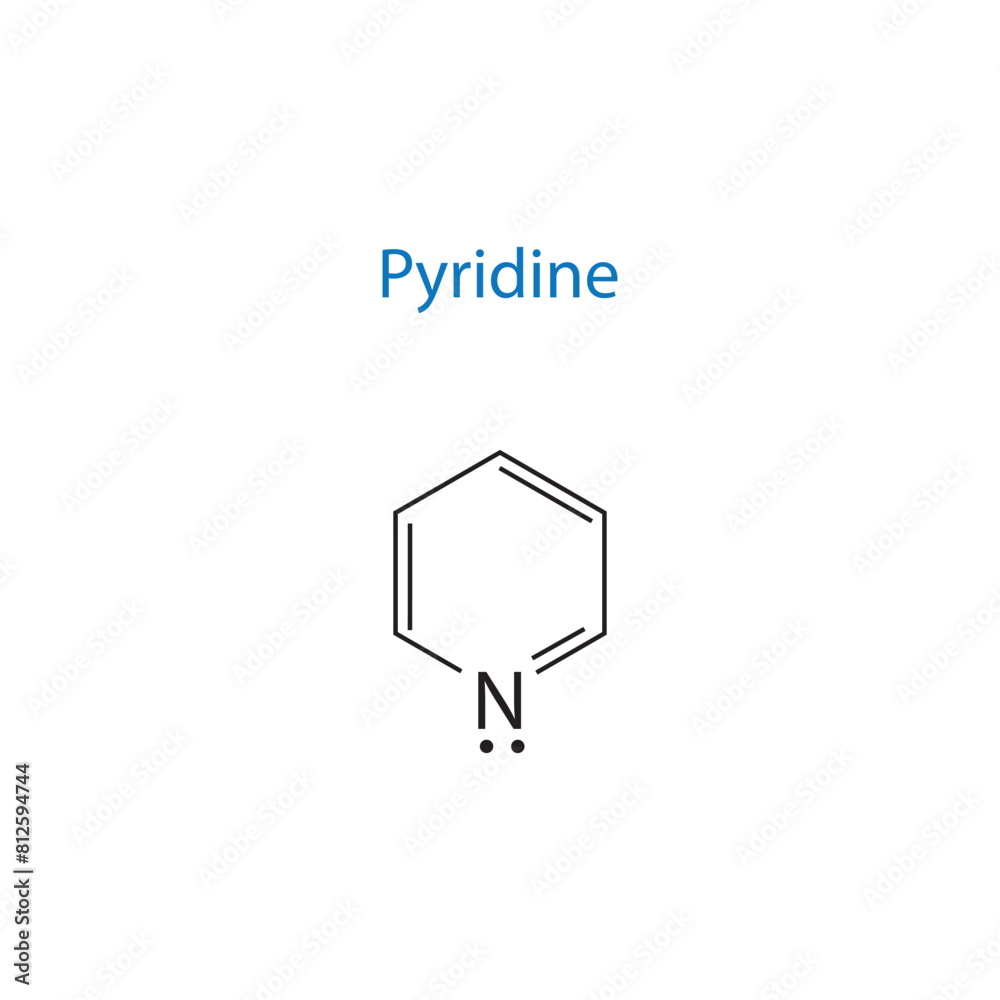
In the vast landscape of organic chemistry, one compound stands out not for its flashy structure or dramatic reactivity, but for its quiet, indispensable role—pyridine. With the formula C₅H₅N, this six-membered heterocyclic aromatic compound forms the backbone of countless pharmaceuticals, agrochemicals, and industrial materials. Though often overshadowed by more viscerally reactive molecules, pyridine’s unique chemical properties make it a linchpin in synthetic pathways, medicinal design, and interdisciplinary science.From stabilizing catalyst ligands to enabling life-saving drugs, pyridine’s influence is deep, enduring, and profoundly practical. Its journey from a curiosity in early organic studies to a cornerstone of industrial chemistry underscores both its versatility and the precision required in its handling and application.
Chemical Structure and Core Properties Pyridine’s structure consists of a planar ring where five carbon atoms alternate with one nitrogen atom at the 1-position, giving it aromatic stability despite the heteroatom’s differing electron configuration. The nitrogen lone pair lies in an sp² hybrid orbital perpendicular to the ring, contributing to pyridine’s basicity—similar to benzene but with enhanced nucleophilic character.
Unlike benzene, pyridine exhibits moderate polarity, enabling solubility in polar solvents and participation in hydrogen bonding. Its freezing point (-105°C) and boiling point (228°C) reflect a balance of intramolecular forces, avoiding the excessive volatility of alkenes while resisting the rigidity of fully saturated systems. This unique blend of stability and reactivity positions pyridine as both a robust scaffold and a responsive intermediate in chemical synthesis.
Synthesis: From Laboratory Concept to Industrial Scale The origins of pyridine date back to 1823, when German chemist Friedrich August Knorig synthesized it via the condensation of acrolein and ammonia, a method later refined into the Bönnemann cyclization and the more modern Chichibabin pyridine synthesis.
Modern production hinges on three main routes:
1. Coordination-Emplication Synthesis
Catalytic methods using transition metals like cobalt or nickel enable efficient ring closure from aldehydes and ammonia, minimizing toxic byproducts.2.
Catalytic Cyclization Recent advances leverage palladium- or rhodium-based catalysts to form pyridine rings via C–H activation, offering cleaner, more atom-economical paths.
3. Biocatalytic Routes
Emerging enzymatic processes harness engineered microorganisms to produce pyridine from renewable feedstocks, aligning with green chemistry principles.Industrial output exceeds thousands of tons annually, driven by demand in pharmaceuticals, agrochemicals, and specialty materials. Scalable, sustainable synthesis remains a focal point, ensuring supply without compromising environmental standards.
Pharmaceutical Significance: Building Blocks of Life-Saving Drugs
Pyridine’s true power lies in its role as a molecular scaffold for pharmaceuticals. Its rigidity and basic nitrogen center enhance drug-target interactions, improving binding affinity and metabolic stability.
Over 20% of FDA-approved drugs feature pyridine rings, integrated into anti-inflammatory agents, antiviral compounds, and treatments for neurodegenerative disorders.
1. Anti-Infectives and Antibiotics
Pyridine derivatives like nicotinamide are precursors to critical coenzymes, while compounds such as pyridomycins act as antibacterial agents by disrupting essential cellular processes.2. Neurological and Psychiatric Therapies
Drugs including vortioxetine—used for major depressive disorder—rely on pyridine-based frameworks to modulate serotonin receptors with precision. The ring’s electron-delocalization enhances bioavailability and selectivity.3. Oncological Applications
Pyridine-containing molecules such as erlotinib, a tyrosine kinase inhibitor, exemplify how this heterocycle targets cancer pathways. Its incorporation boosts drug half-life and target specificity, accelerating therapeutic efficacy.Each pharmaceutical use leverages pyridine’s structural integrity and tunable electronic properties, proving its irreplaceability in modern medicine.
Industrial Applications Beyond Medicine
Beyond pharmaceuticals, pyridine underpins critical sectors including agrochemicals, polymers, and specialty additives. Its derivatives act as herbicides, insecticides, and plant growth regulators, increasing agricultural yields safely. In materials science, pyridine-based ligands stabilize metal complexes used in OLEDs and catalytic converters, driving advancements in electronics and green technology.
Agrochemical Uses
1. Herbicides and Pesticides
Pyridinecarboxylic acids form the core of potent herbicides like picloram, effective against broadleaf weeds while minimizing crop damage.2.
Insect Propellants Certain pyridine derivatives serve as synergists, enhancing pesticide potency and reducing required dosages.
In industrial polymer chemistry, pyridine functions as a stabilizer in polyamides and polyimides, extending material lifespan under extreme conditions. Its role in ligand design also enables efficient catalysts, crucial for fine chemical synthesis and green manufacturing processes.
Safety and Handling: Managing Pyridine’s Reactivity Though indispensable, pyridine demands careful handling due to its toxicity, flammability, and potential for irritation.
It is classified as a hazardous substance with exposure risks through inhalation, skin contact, or ingestion. The U.S. OSHA limits airborne exposure to 1 ppm over an 8-hour shift, while proper PPE—gloves, goggles, respirators—is mandatory in labs and factories.
Storage requires sealed containers in well-ventilated areas, isolated from strong acids and oxidizers. In case of spillage, absorbent materials paired with neutralization protocols prevent environmental contamination. Ongoing research into safer pyridine analogues aims to reduce health risks while preserving functional advantages.
Analytical Methods: Detecting and Characterizing Pyridine Reliable detection is essential across applications.
Standard techniques include:
1. Gas Chromatography-Mass Spectrometry (GC-MS)
Widely used for purity analysis, GC-MS identifies trace contaminants in pharmaceuticals and environmental samples with high sensitivity.2.
Nuclear Magnetic Resonance (NMR) Spectroscopy ¹H-NMR reveals ring proton environments, confirming structure and quantifying regioselectivity—critical for quality control.
3. UV-Vis Spectroscopy
Used in real-time process monitoring, its characteristic absorption aids in tracking pyridine in reaction mixtures and industrial streams.These tools ensure pyridine’s integrity in production, research, and regulatory compliance, safeguarding both product efficacy and safety.
Environmental and Sustainability Considerations
With increasing focus on sustainability, pyridine’s lifecycle is under scrutiny. Its production, historically reliant on petrochemicals, is shifting toward biobased routes using renewable feedstocks like biomass-derived acrolein and ammonia. Such transitions reduce carbon footprints and dependence on fossil resources.
Additionally, advances in catalytic pyridine synthesis aim to cut energy use and waste generation, aligning with green chemistry mandates. Regulatory bodies monitor industrial effluents closely, requiring stringent waste treatment to prevent soil and water contamination. Responsible handling and disposal—including closed-loop systems and biodegradability studies—are now central to pyridine’s modern industrial profile, ensuring its long-term viability in a circular economy.
Ongoing Research and Future Frontiers
Pyridine remains a vibrant area of scientific inquiry, with researchers probing novel uses and sustainable production.
Key insights from recent years include:
1. Enhanced Drug Design
Computational modeling and high-throughput screening accelerate the discovery of pyridine derivatives with improved pharmacokinetics and reduced side effects.2.
Biocatalytic Innovation Engineered enzymes now enable selective pyridine alkylation and N-functionalization, minimizing reagent use and expanding synthetic flexibility.
3. Sustainable Feedstocks
Plant-derived precursors and electrochemical synthesis promise cleaner, scalable pathways, reducing reliance on nonrenewable resources.These advances ensure pyridine evolves alongside global scientific and environmental priorities, maintaining its status as a key player in organic chemistry.
Conclusion
Pyridine, though often overlooked, is a silent architect shaping the modern world—from life-saving medications to high-performance materials and agricultural essentials. Its unique aromatic stability, basic character, and synthetic versatility underpin innovations across chemistry, medicine, and industry.
With progress in sustainable synthesis, precision medicine, and green manufacturing, pyridine continues to meet the demands of science and society alike, proving that the most crucial compounds are often those quietly driving change beneath the surface.

Related Post
Enedina Arellano Felix Today: A Deep Dive Into the Life of a Crimson Trailblazer in Mexico’s Infamous Criminal History
Gypsy Rose Crime Scene Photos Reveal the Shocking Truth Behind a National Infamy

Kate Hudsons Wealth Revealed: Decoding the Business Acumen and Investment Strategy Behind a Rising Financial Powerhouse
Shaquille O’Neal: Beyond the Altura—A Title of Imagination and Reality

