Products And Reactants Of Aerobic Respiration
Products and Reactants of Aerobic Respiration: The Precise Molecular Recipe Driving Life’s Energy Engine — Aerobic respiration stands as one of biology’s most fundamental processes, underpinning energy production in nearly all eukaryotic life forms. At its core, this metabolic powerhouse relies on a tightly choreographed sequence of chemical reactions, where specific reactants fuel a cascade yielding key products essential for cellular function. Understanding the precise reactants consumed and the products generated offers not only insight into cellular energetics but also a window into the biochemical elegance sustaining life.
From glucose’s role as primary fuel to oxygen’s indispensable contribution and the vital output of ATP, carbon dioxide, and water, every component plays a non-negotiable part in the respiration equation.
Core Reactants: The Starting Materials Fueling Energy Extraction
The principal reactants in aerobic respiration form the foundation of the metabolic pathway. To ignite this process, three primary inputs are required: a readily available energy store—most commonly glucose—oxygen, and cellular cofactors that facilitate electron transfer. Glucose (C₆H₁₂O₆) serves as the central carbohydrate reactant, supplying the bulk of electrons needed to generate energy.In eukaryotes, glucose enters via transport proteins in cell membranes, typically after dietary carbohydrates are broken down. Its three-carbon structure, after initial processing, becomes central to the glycolysis phase where adenosine triphosphate (ATP) and high-energy electrons are captured. Oxygen (O₂) functions as the final electron acceptor in the electron transport chain (ETC), a critical step enabling the bulk production of ATP.
Without oxygen, the entire oxidative phosphorylation mechanism stalls, halting energy output in most aerobic organisms. Coenzymes such as nicotinamide adenine dinucleotide (NAD⁺), flavin adenine dinucleotide (FAD), and coenzyme Q are not consumed but serve as vital carriers of electrons, shuttling energy between reaction stages. These molecular partners ensure the smooth flow of redox reactions, making them indispensable reactants despite their transient use.
Each reactant’s availability directly impacts metabolic efficiency; disruptions in glucose supply or oxygen access can rapidly degrade cellular performance, highlighting the delicate balance required for sustained respiration.
Elephants in the Biochemical World: Key Products Released
The products of aerobic respiration reveal the process’s dual role: extracting maximal energy while managing safe waste. The three principal outputs—ATP, carbon dioxide (CO₂), and water (H₂O)—are not mere byproducts but essential byproducts of efficient cellular oxidation. ATP serves as the primary energy currency of the cell.Though only ~30–32 ATP molecules are generated per glucose under ideal conditions via oxidative phosphorylation, this energy powers nearly every cellular function, from muscle contraction to active transport. Each phosphate bond represents a stored energy packet, extracted through the controlled breakdown of the glucose molecule and facilitated by oxygen-dependent ETC activity. Carbon dioxide is released into the environment as CO₂, a waste gas produced during the decarboxylation steps of the citric acid cycle (Krebs cycle).
Despite being a byproduct, CO₂ is vital for ecosystem-scale carbon cycling. Plants and photosynthetic organisms later reuse this gas, closing the loop in nature’s metabolic design. Water forms as oxygen accepts electrons and combines with hydrogen ions generated in earlier stages.
This reaction is catalyzed by cytochrome c oxidase in the mitochondrial inner membrane, ensuring complete proton transfer and oxygen utilization. The water produced is critical for maintaining proton gradients essential to ATP synthesis.
Together, these products illustrate aerobic respiration’s role: not just energy generation, but a balanced exchange critical to organismal survival and environmental dynamics.
Mapping the Metabolic Pathways: Reactants Transformed into Vital Products
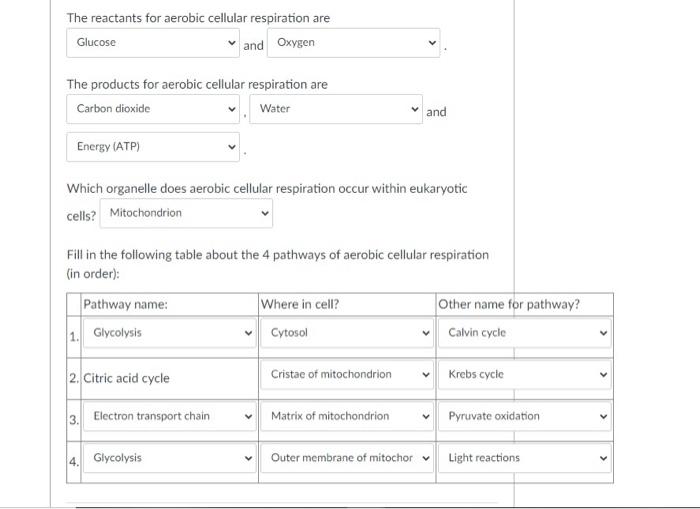
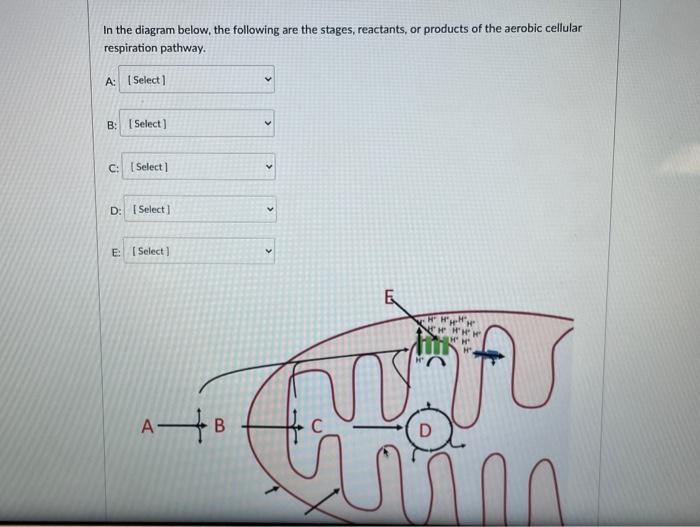
Aerobic respiration unfolds through four interconnected stages—glycolysis, pyruvate oxidation, the citric acid cycle, and oxidative phosphorylation—each transforming reactants into products with precision.- **Glycolysis** begins with glucose near the process’s doorstep. Though it consumes no external oxygen, this cytoplasmic event splits glucose into two pyruvate molecules while generating a small gain of 2 ATP and 2 NADH—molecules carrying high-energy electrons. - **Pyruvate Oxidation** transitions pyruvate into acetyl-CoA, releasing one CO₂ per pyruvate and generating one NADH, readying substrates for the Krebs cycle.
- The **citric acid cycle** amplifies energy extraction: each acetyl-CoA combusts completely, producing three NADH, one FADH₂, and one ATP per turn, alongside two CO₂ emissions—accumulating from multiple turns to fuel over 20 NADH and FADH₂ molecules total. - **Oxidative Phosphorylation** is where the bulk of energy is harvested. Electron transport chain complexes institutionalize electron flow, using NADH and FADH₂ to pump protons across the inner mitochondrial membrane.
This creates a gradient, driving ATP synthase to generate ~26–28 ATP. Oxygen acts as the terminal electron acceptor, combining with protons and electrons to form water—an elegant cleanup that prevents toxic buildup.
Each stage depends on precise reactant interactions: glucose fuels glycolysis; NAD⁺ and FAD accept electrons; oxygen prevents stalling; and ATP synthase converts proton motive force into chemical energy.
![[Solved]: reactants for aerobic cellular respiration are an](https://media.cheggcdn.com/study/9d3/9d3229c5-0150-4b3a-878c-81ca8a1bd717/image)

Related Post

Arlene Martel: From Stage Whispers to Cinematic Immortality

Zara Online Returns: Your Step-by-Step Guide to Hassle-Free Exchanges & Refunds
Is Tom Hanks A Pedophile? Unraveling The Truth Behind The Explosive Allegations

Brooke Silverang Married: Navigating Love, Career, and Commitment in the Public Eye

