Processes Packages Proteins and Lipids in Vesicles: The Cellular Machinery Behind Exported Cargo
Processes Packages Proteins and Lipids in Vesicles: The Cellular Machinery Behind Exported Cargo
At the heart of cellular logistics lies a complex, highly orchestrated system: the regulated export of proteins and lipids via specialized vesicles. These microscopic transporters, enclosed by lipid bilayers and precisely packed with cargo, navigate a labyrinth of intracellular pathways to deliver essential molecules to destinations both inside and outside the cell. Central to their function are the tightly regulated processes that manage the assembly, cargo sorting, membrane fusion, and distribution—processes driven by sophisticated packages of proteins and lipids that ensure accuracy, efficiency, and safety.
Understanding how these molecular components collaborate reveals the elegance of cellular export mechanisms and their vital role in health and disease.
Sorting and Packaging: The Organelle-Specific Protein Machinery
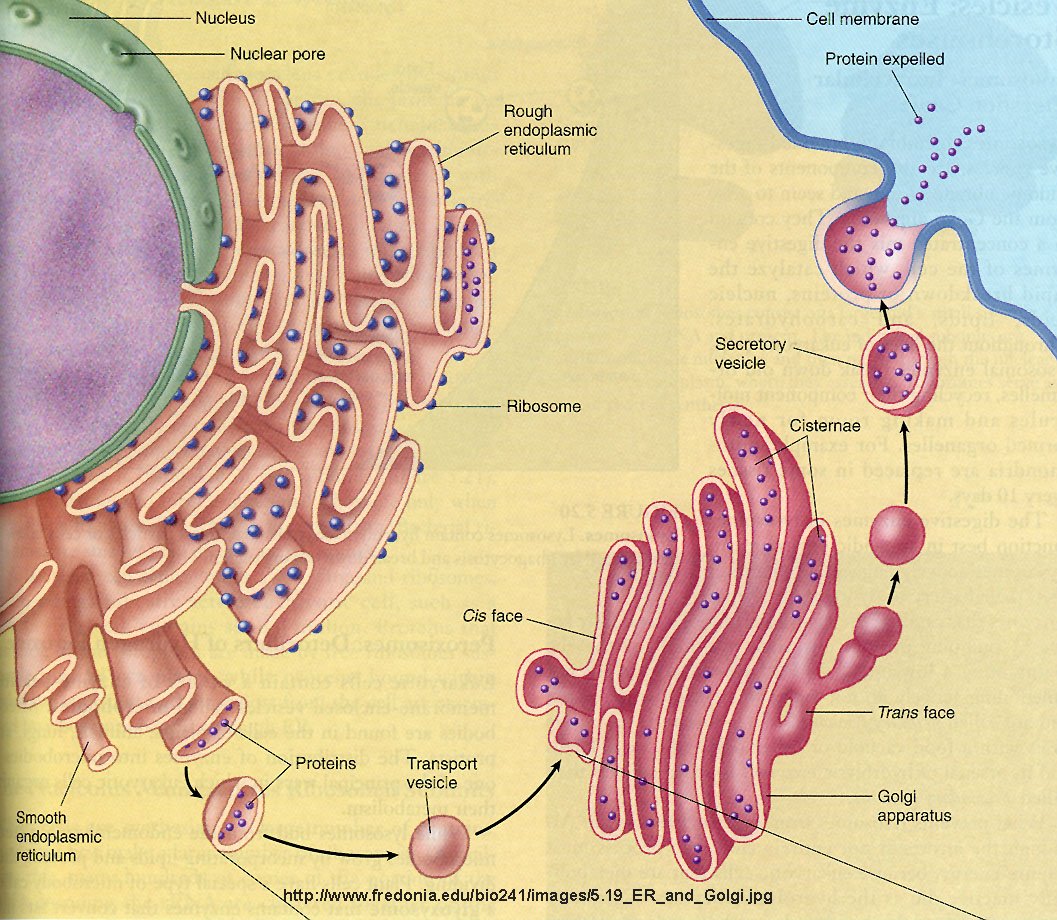
Each vesicle type is defined by its targeting specificity, established during sorting in donor organelles such as the endoplasmic reticulum (ER), Golgi apparatus, endosomes, and clathrin-coated pits. A diverse array of sorting receptors and coat proteins ensures only the correct cargo is selected.For proteins destined for secretion, the disibration complex—comprising SV2, STX (Syntaxin family), and Sec5—plays a critical role in mediating cargo selection at the Golgi. These proteins recognize specific signal sequences, such as sorting signals in the C-terminal regions of pre-secretory proteins, guiding them into transit vesicles. “Sorting is not random—each protein and lipid is ESI-ed into the right vesicle by a coded language of motifs and adaptors,” explains molecular biologist Dr.
Elena Marquez. “Molecules bind preciously to coat proteins, which act as molecular scissors and acceptors, ensuring fidelity.” This specificity prevents misrouting, which could disrupt cell signaling, immune function, or nutrient transport. In lipid transport, specialized carriers like lipoprotein particles assemble with specific phospholipids and cholesterol, facilitated by lipid transfer proteins (LTPs) and donor protein scaffolds.
- **Vesicle Identity** comes from coat proteins: COPI facilitates retrograde ER-to-Golgi transport; COPII drives anterograde trafficking from ER to Golgi. - **Cargo Selection** depends on sorting signals—such as di-acidic sequences in clathrin-mediated endocytosis or glycosylation patterns on glycoproteins. - **Sorting Complexes** like the AP-1 complex at the trans-Golgi node ensure forward trafficking to the cell membrane.
Lipids themselves are not passive bystanders. Their composition dynamically influences vesicle identity and fusion. For example, phosphatidylinositol 4-phosphate (PI(4)P) enriched at Golgi membranes recruits efflux complexes, while sphingomyelin and cholesterol modulate curvature and fusion competence.
This lipid-protein interplay creates microdomains essential for efficient vesicle budding and docking.
Membrane Fusion: The Final Step of Export Control
Once a vesicle is cargo-loaded and targeted, it must unlock the gate to its destination—a process governed by SNARE proteins. The vesicle membrane’s v-SNAREs dock with target-tethering complexes on the plasma membrane, most notably the t-SNARE syntaxins and SNAP-25s.This pairing forms a tight SNARE complex that drives membrane fusion. “Fusion is a molecular handshake—precision at every step,” notes xenobiologist Dr. Rajiv Patel.
“SNAREs must align perfectly to avoid misfusion, ensuring cargo reaches the right membrane ‘door.’” Cofactors such as synaptotagmin act as calcium sensors, linking vesicle exocytosis to signaling triggers like action potentials or calcium influx. This ensures secretory vesicles fuse only when and where needed. In lipid export, proteins like ORP (Oxysterol-binding protein-related) families mediate lipid transfer across membranes, maintaining lipid homeostasis between organelles.
The fusion event itself triggers lipid redistribution and protein recycling, closing the export loop seamlessly. The whole process is exquisitely regulated by small GTPases—Rab proteins—steering vesicle movement and tethering. Rab GTPases bind to specific effectors, enabling stepwise progression: Rab1 coordinates ER-Golgi intermediates; Rab27 directs melanosome and lysosome trafficking.
Each step is checkpoint-controlled, preventing premature fusion or cargo release.
Lipid Dynamics: More Than Structural; Functional Drivers of Export
Lipids in export vesicles do far more than supply membrane structure—they actively govern vesicle biogenesis, cargo retention, and fusion. Cholesterol, for example, stabilizes domains critical for SNARE complex assembly.Phosphatidylserine exposure on inward-facing vesicle membranes signals targeting and fusion readiness. Modifications such as palmitoylation and myristoylation anchor proteins to specific membrane regions, increasing functional efficiency. “Lipids sculpt the vesicle’s identity just as profoundly as proteins,” asserts lipid biologist Dr.
Priya Nair. “How lipids cluster, saturated vs. unsaturated ratios, even local charge effects—all influence which molecules are packed and where they go.” Recent advances show lipid metabolism enzymes, such as scramblases and flippases, actively shape vesicle membrane composition *en route*.
This active remodeling ensures cargo remains segregated until fusion, minimizing leakage or cross-talk. In immune cells, lipid redistribution into secretory vesicles supports antigen presentation and cytokine release. In neurons, lipid-rich secretory vesicles maintain synapse integrity and neurotransmitter delivery.
In disease states—such as neurodegenerative disorders or cancer—disruptions in lipid-protein interactions lead to export failures: misfolded proteins accumulate, membranes destabilize, and signaling breaks down. Understanding these mechanisms not only illuminates cellular function but informs therapeutic strategies aimed at restoring exocytic fidelity. This intricate choreography—where proteins package cargo, lipids fine-tune dynamics, and regulatory GTPases direct movement—represents one of biology’s most elegant transport systems.
It stands as a testament to nature’s precision: every exported vesicle is a molecular courier, guided by an evolved package of proteins and lipids, delivering life’s essential messages across cellular boundaries.