One Proton Defines Lithium’s Spark: The Atomic Heart Behind Earth’s Most Dynamic Element
One Proton Defines Lithium’s Spark: The Atomic Heart Behind Earth’s Most Dynamic Element
Lithium, with its singular proton core, stands as a quiet powerhouse in the realm of chemistry and technology. As the third-lightest element and the first in the alkali metal series, lithium’s identity is rooted in its atomic structure: exactly one proton in its nucleus. This single nucleon sets the stage for a cascade of unique chemical and physical properties that make lithium indispensable in modern life—from mobile batteries to life-saving medical treatments.
Understanding the role of a single proton reveals not just a number, but a foundation for innovation.
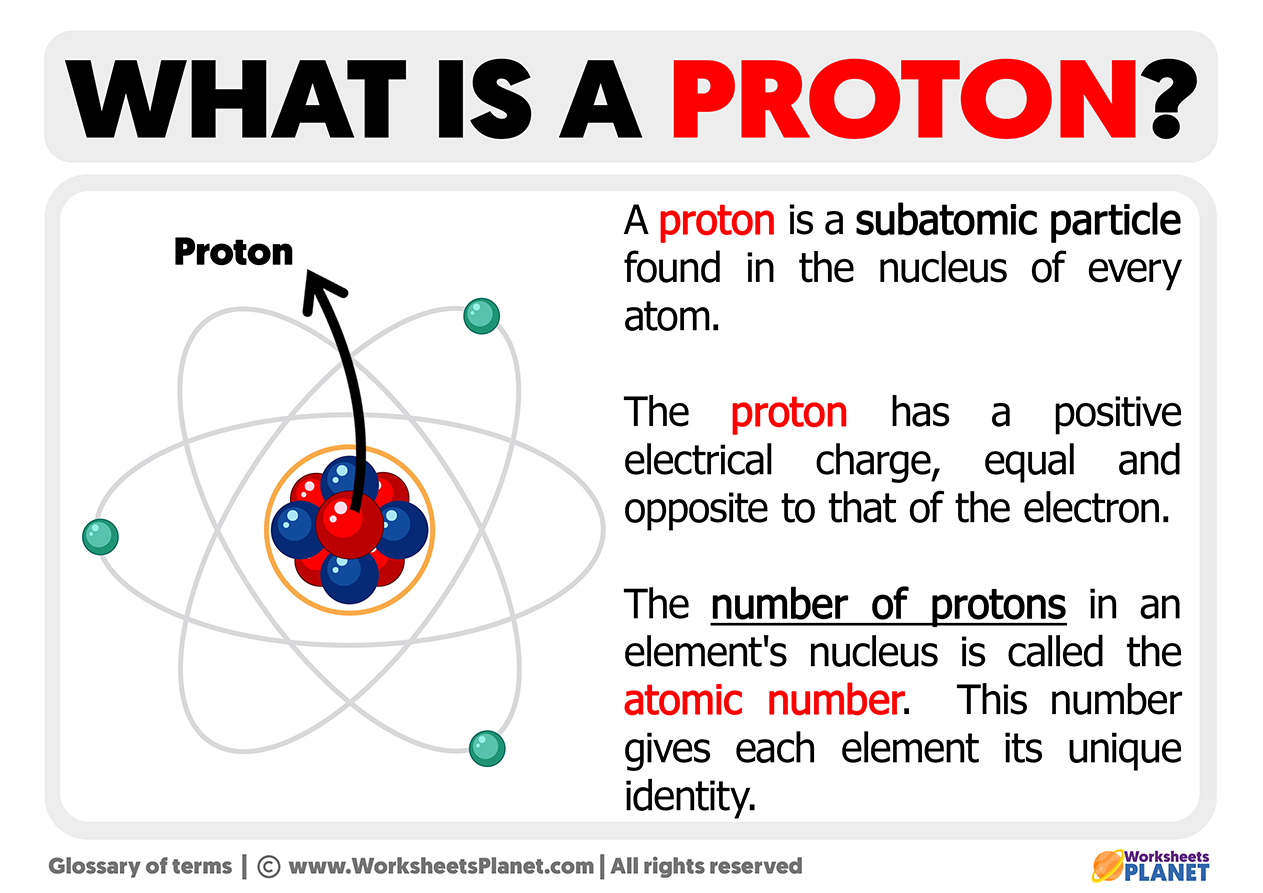
At the heart of lithium’s atomic number lies a deceptively simple truth: it contains precisely one proton. This defining feature determines lithium’s position on the periodic table and fundamentally shapes its behavior.
The proton count establishes lithium’s atomic mass (~6.94 atomic mass units), defines its electron configuration, and dictates how it interacts in chemical bonds. In lithium, the nucleus holds one positively charged proton surrounded by a cloud of electrons, creating the element’s signature reactivity. “Hydrogen has one proton, lithium has one—but in quite a different chemical world,” notes Dr.
Elena Torres, a quantum chemist at Stanford University. “That single proton unlocks lithium’s ability to release energy so efficiently, a trait leveraged in cutting-edge technologies.”
To grasp lithium’s significance, consider its number of protons in context. With atomic number 3, lithium belongs to a family of elements—beyond hydrogen and helium—where the proton count grows steadily through the periodic table.
Its first proton establishes the element’s basic structure, enabling the formation of ionic compounds like lithium chloride and lithium hydroxide. These compounds power countless applications: from desalination and water purification to advanced energy storage systems. “Each proton sets the stage for lithium’s flexibility,” explains Dr.
James Chen, materials scientist at MIT. “With just one, lithium avoids rigid bonding tendencies seen in heavier metals, allowing for dynamic ion movement—especially crucial in batteries.”
What makes lithium distinct—and vital—is how its solitary proton facilitates a delicate balance of instability and utility. Unlike elements with multiple protons that often form complex, inert configurations, lithium’s single proton fosters an electron shell that readily relinquishes energy upon ionization.
When lithium loses one electron, forming lithium ions (Li⁺), it releases usable energy—harnessed in everything from smartphone batteries to grid-scale storage. “A single proton fuels lithium’s remarkable ability to store and release electrical charge efficiently,” adds Dr. Torres.
“This efficiency is why lithium-ion batteries have revolutionized portable electronics and electric vehicles.”
The Role of Protons in Lithium’s Chemical Behavior
• **Electron Configuration**: Lithium’s electron arrangement follows the pattern 1s² 2s¹, with the single 2s electron available for chemical reactions—explaining its high reactivity. • **Ionization Dynamics**: With only one proton and one electron loosely bound, lithium easily donates its electron, a behavior central to its use in redox reactions. • **Alloying and Compound Formation**: The single proton allows lithium to form stable yet reactive alloys with metals like aluminum or nickel—key components in lightweight, high-performance batteries.• **Battery Efficiency**: During discharge, lithium’s androidic flight—its proton-centered electron flow—enables rapid energy transfer with minimal loss.
In every application, from the micro-scale of battery electrodes to the macro-scale of renewable energy grids, lithium’s identity hinges on that one proton. This elementary particle is not just a number, but a symbol of efficiency, adaptability, and innovation.
It defines lithium’s place in chemistry, sets the stage for its technological prominence, and illuminates the profound impact of atomic structure on human advancement. As demand for clean energy and portable electronics grows, the significance of lithium’s singular proton only deepens—reminding us that sometimes, the smallest components hold the greatest power.



Related Post

PCN Login: Your Gateway to Financial Empowerment in the Digital Banking Era

Unlocking Next-Generation Performance: How Intel Newsletters Bf6 Shape the Future of Computing
Natalie Walters Meteorologist Bio Wiki Age Husband Salary and Net Worth

