Number Of Protons in Gold: The Atomic Secret Behind This Precious Metal
Number Of Protons in Gold: The Atomic Secret Behind This Precious Metal
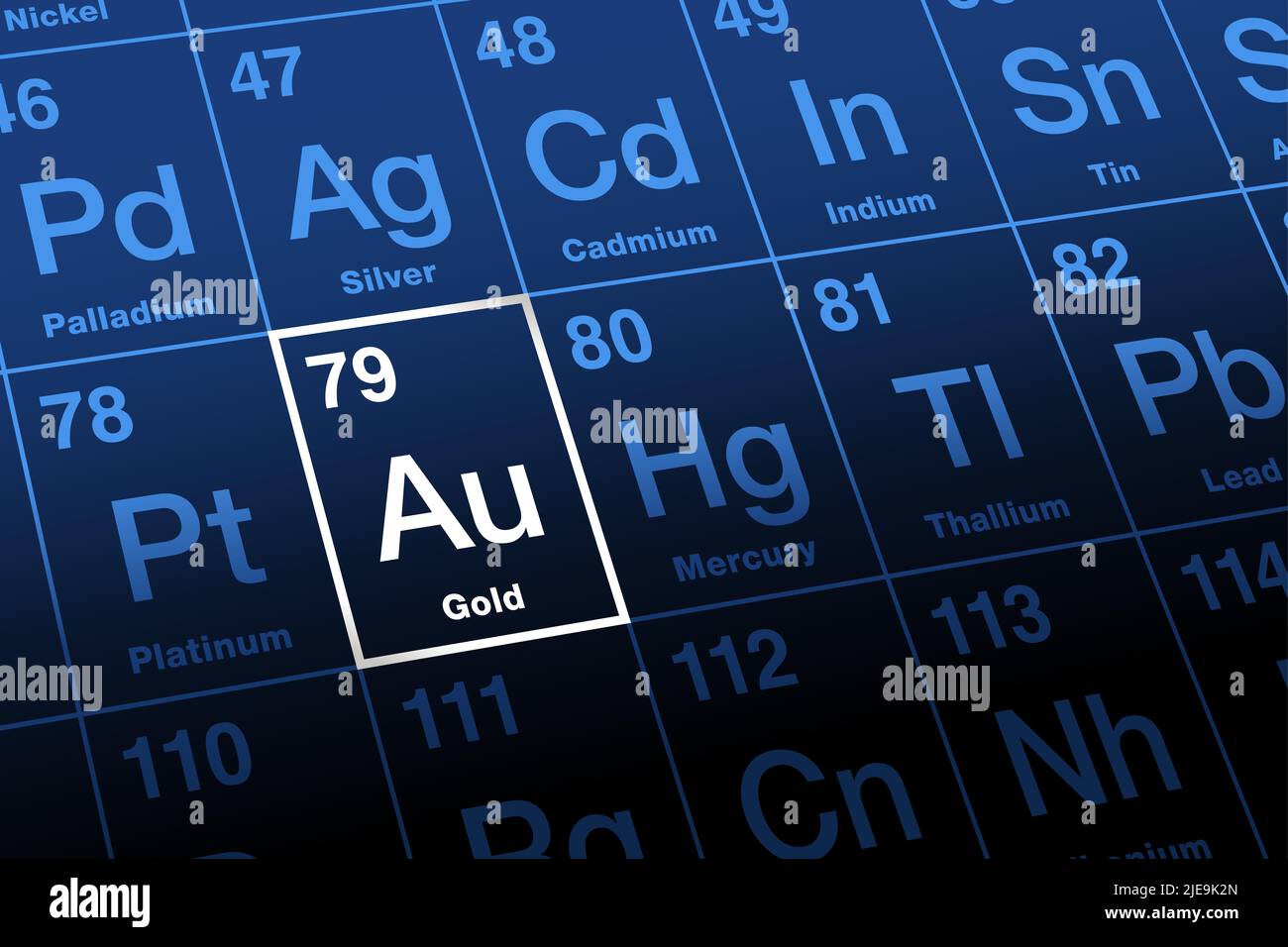
Gold, with its timeless luster, cultural significance, and role as a financial safe-haven asset, has long captivated scientists and scholars alike. Yet beneath its glittering surface lies a fascinating nucleus: the precise number of protons in gold atoms, a key determinant of its chemical identity and behavior. With precisely 79 protons, gold’s atomic number defines not only its elemental identity but also influences its stability, reactivity, and place in the periodic table.
Understanding this number unlocks deeper insight into why gold remains both unique and indispensable—from ancient jewelry to cutting-edge technology. Number 79: The Proton Count That Defines Gold The proton number, or atomic number, is the fundamental marker of elemental identity. In gold (Au), this number stands unwavering at 79.
Every gold atom possesses exactly 79 protons in its nucleus, a quantity that distinguishes it from all other elements and governs its electron configuration, chemical interactions, and physical traits. This specific count places gold in Group 11 of the periodic table, alongside copper and silver, within the transition elements that shape modern materials science. Unlike elements with rounded atomic numbers—such as arsenic (33) or cerium (58)—gold’s modest sum of 79 protons results in a refined electron arrangement.
The atomic electron configuration of gold reflects a notable stability: the 4f and 5d electron shells are nearly full, minimizing reactivity and contributing to gold’s well-known resistance to corrosion. "With 79 protons providing a balanced nucleon-proton ratio, gold achieves a rare combination of durability and workability," explains Dr. Elena Marquez, a materials chemist at the Institute of Advanced Metallurgy.
"This stability explains why gold survives millennia in artifacts and remains reliable in everything from electronics to gold leaf." Seeing 79 protons is more than counting atoms—it reveals gold’s quantum signature. Each proton contributes about two-thirds of the atom’s mass, anchoring gold’s position at approximately 196.97 atomic mass units. This mass arises from a careful sum of protons and neutrons, with gold typically featuring around 118 to 120 neutrons to maintain nuclear equilibrium.
These precise nuclear details underscore gold’s atomic authenticity, making isotopic analysis a powerful tool in verifying purity and provenance.
The Role of 79 Protons in Gold’s Chemical Stability
Gold’s atomic number of 79 is not merely a numerical footnote—it directly influences its chemical behavior. The 79 protons create a nucleus that binds 79 electrons with extraordinary cohesion, producing a stable valence shell configuration that defies rapid oxidation or degradation.This stability is what allows gold to excel in environments where other metals fail: marine chambers, industrial catalysts, and high-temperature electronics alike. While gold’s low susceptibility to chemical reactions stems largely from its full outer electron shell, the underlying proton count ensures predictable electron delocalization and strong metallic bonding. Unlike paramagnetic or highly reactive elements, gold’s 79-proton nucleus supports a nearly spherical electron probability distribution, reinforcing its luster and conductivity.
"The exact proton number ensures gold maintains a consistent, resilient surface layer—resistant both to tarnish and to sputtering under electron beams," notes Dr. Klaus Weber, a nuclear physicist specializing in heavy elements. This resistance is experimentally verified: gold does not oxidize in air at room temperature and resists corrosion even under extreme photonic exposure.
These traits make 79 protons not just a scientific curiosity, but a cornerstone of gold’s utility in everything from microelectronics to biomedical implants.
Proton Count vs. Everyday Perception: Why 79 Matters
In popular discourse, gold is often perceived through the lens of value, history, or jewelry—Less frequently, through its atomic structure.Yet the count of 79 protons quietly shapes these narratives. Gold’s atomic order within the periodic table explains why it holds its place between silver (47 protons) and mercury (80), forming a transitional link in the d-block that balances reactivity and durability. This positioning also influences how gold interacts with light.
Its distinctive yellow hue, arising from relativistic electron effects amplified by the 79-proton nucleus, is a direct consequence of subtle energy shifts in electron transitions. Such color properties, while visually striking, are rooted in atomic precision—making every visible gold object a tangible expression of nuclear architecture. Moreover, the proton number aids scientific analysis and quality control.
In refining processes, mass spectrometry and neutron activation rely on precise atomic signatures tied to 79 protons. This enables forensic authentication of gold purity, detection of alloys, and verification of historical artifacts—underscoring how elemental numbers translate into real-world applications.
The Proton Number as a Gateway to Innovation
Beyond identity, gold’s 79 protons fuel technological advancement.In semiconductors, gold’s surface plasmon resonance—governed by its electron sea, shaped by atomic protons—enables ultra-sensitive sensors and adaptive optics. In catalysis, gold nanoparticles leverage their electron density, fine-tuned by the 79-proton core, to accelerate reactions critical in green chemistry and pollution control. Nuclear physics further reveals gold’s unique position: with 79 protons, gold exhibits stable isobars rarely found in nature, making it a model compound for studying nuclear shell effects and neutron capture.
Its use in medical imaging, particularly in gold nanoparticle contrast agents, exploits its inertness and optical properties—both traceable to its atomic foundation. As research progresses, the 79-proton signature of gold continues to inform the design of new alloys, quantum materials, and sustainable technologies. Scientists increasingly recognize that elemental numbers are not abstract data points, but blueprints for innovation.
Gold’s identity—defined by precisely 79 protons—epitomizes how atomic structure shapes macroscopic reality. This number governs its electron arrangement, chemical inertness, and optical brilliance, transforming gold from a symbol of wealth into a material of scientific wonder. From ancient civilizations to modern labs, the proton count reveals gold’s enduring legacy: not by chance, but by design, deep in the nucleus.
Every time gold shines, it carries forward an atomic truth—less than 80 protons, but infinitely significant.



Related Post
Thea Hail Using Exact Entrance Theme Song as Impact Wrestling Star
Star Rangers: Commanding the Stars with Precision and Power

Black Hat Baseball in the Digital Realm: Fact or Fiction in the “Fact or Fiction in a Gooogle Doodle”?

