Nitrogen Molar Mass: The Invisible Backbone of Chemistry’s Most Everyday Environment
Nitrogen Molar Mass: The Invisible Backbone of Chemistry’s Most Everyday Environment
With an atomic weight that anchors the foundation of life, nitrogen stands as one of the most abundant yet underappreciated elements in Earth’s atmosphere. Representing 78% of dry air, nitrogen’s molar mass—approximately 28.0134 grams per mole—serves as a silent sentinel in pharmacology, agriculture, atmospheric science, and materials engineering. Its predictable stoichiometry enables precise measurements crucial to scientific progress, while its inert nature ensures stability across industries.
Understanding nitrogen’s molar mass is not just an academic exercise—it’s essential to mastering the chemistry that shapes modern life.
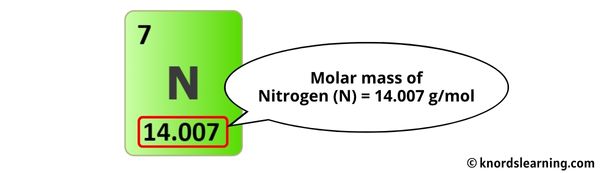
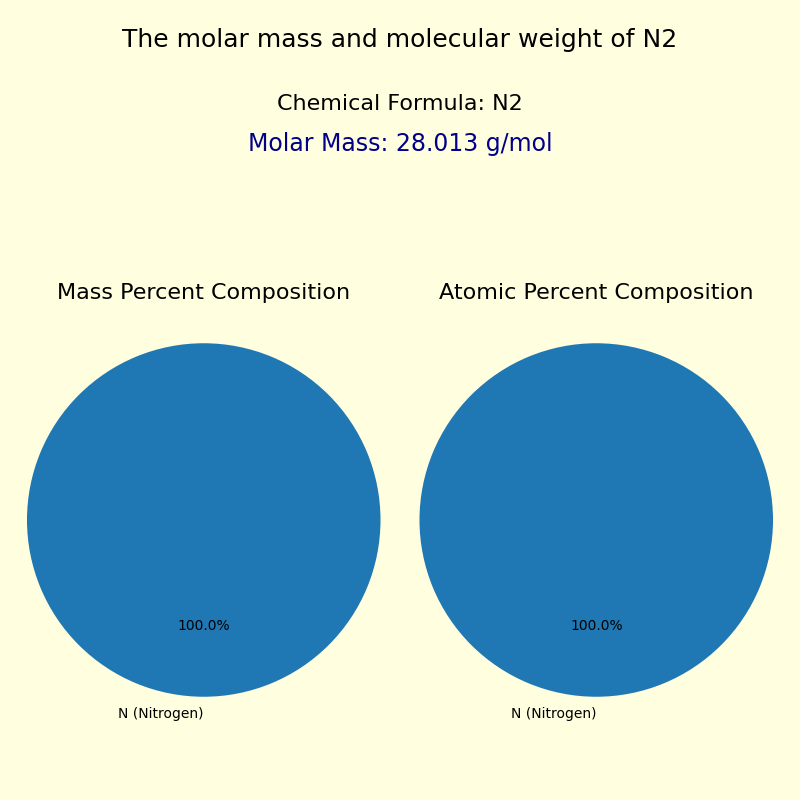
Nitrogen’s designation as a diatomic element—N₂—underpins its molar mass. Each nitrogen atom contributes roughly 14 atomic mass units (amu), resulting in a diatomic mole weighing exactly 28.0134 g/mol when rounded to four significant figures.
This value, derived from the weighted average of naturally occurring isotopes—primarily N-14 (99.6%) and a trace of N-15 (0.4%)—empowers analysts from chemists to environmental scientists with a reliable standard.
The precision of nitrogen’s molar mass enables reliable analytical techniques.
High-accuracy methods such as gas chromatography, mass spectrometry, and spectroscopic analysis depend on nitrogen’s consistent molar value to detect minute concentrations in complex mixtures. For example, in medical diagnostics, nitrogen-based analysis helps monitor blood gas levels, guiding life-saving interventions.In industrial settings, precise molar measurements ensure batch consistency in the production of fertilizers, pharmaceuticals, and high-purity industrial gases.
Beyond measurement, nitrogen’s molar mass directly influences its behavior in both natural and engineered systems. Its low molecular weight and noble characteristics render it chemically inert, preventing unwanted reactions under standard conditions.
This stability is exploited across multiple sectors. In agriculture, nitrogen’s role in ammonia synthesis—via the Haber-Bosch process—relies fundamentally on controlled stoichiometry governed by molar mass. “The controlled reaction between nitrogen and hydrogen hinges on accurate mole ratios,” explains Dr.
Elena Martinez, a physical chemist at the National Center for Nitrogen Research. “Each mole of N₂ provides fixed reactive capacity, allowing engineers to scale production efficiently and safely.”
The environmental significance of nitrogen’s molar mass extends into atmospheric science, where isotopic ratios serve as climate proxies. Variations in N-15 to N-14 ratios naturally tracked via molar mass ratios provide clues into nitrogen cycling, pollution sources, and ecosystem health.
“Nitrogen isotopes act like fingerprints in the biosphere,” notes Professor James Reed, an expert in environmental chemistry. “By measuring shifts in natural molar mass distributions, we trace human impact—from fertilizer runoff to industrial emissions—adding critical context to climate and pollution studies.” This isotopic analysis supports policy decisions and sustainable resource management.
In the pharmaceutical domain, nitrogen’s predictable molar mass ensures reproducibility in drug development.
Active pharmaceutical ingredients often contain nitrogen-rich moieties, and precise molar measurements guarantee dosage consistency and functional reliability. For instance, in antibiotics like penicillin analogs, nitrogen integration is quantitatively dependent on its molar characteristics, ensuring stability and efficacy across batches. Similarly, in combinatorial chemistry and drug screening, nitrogen’s role in molecular scaffolding is fundamentally informed by exact molar mass data, enabling rapid, high-throughput discovery.
Synthetic chemistry, too, relies on nitrogen’s molar mass to design catalysts, ligands, and polymers. In earthquake-proof materials, nitrogen-doped polymers use molar precision to engineer resilience. In fuel technologies, nitrogen’s inclusion in propellants or as a modifier benefits from heretofore unmatched stoichiometric clarity.
The versatility of nitrogen—rooted in chemistry and quantified by molar mass—exemplifies how fundamental atomic properties drive technological innovation across countless disciplines.
In summary, nitrogen’s molar mass of 28.0134 g/mol is far more than a textbook footnote—it is a cornerstone of precision and predictability in science and industry. From enabling life-sustaining atmospheric balance to powering industrial synthesis and environmental monitoring, nitrogen’s atomic signature guides discovery and application alike.
As global challenges in food security, climate health, and material science intensify, mastering the physics and chemistry of elements like nitrogen remains as urgent as ever. Its molar mass, though seemingly abstract, stands as a pillar of the visible and invisible frameworks shaping our world.

Related Post
Apple Account Login: The Fully Secure Gateway to Your Digital Lifeworld

When PSN Falters: Is Your PlayStation Network Down? Here’s How to Check and Respond

From British Pages to Indian Screens: The Legacy of Harry Potter & Deathly Hallows Part 1 in Hindi Cinema

