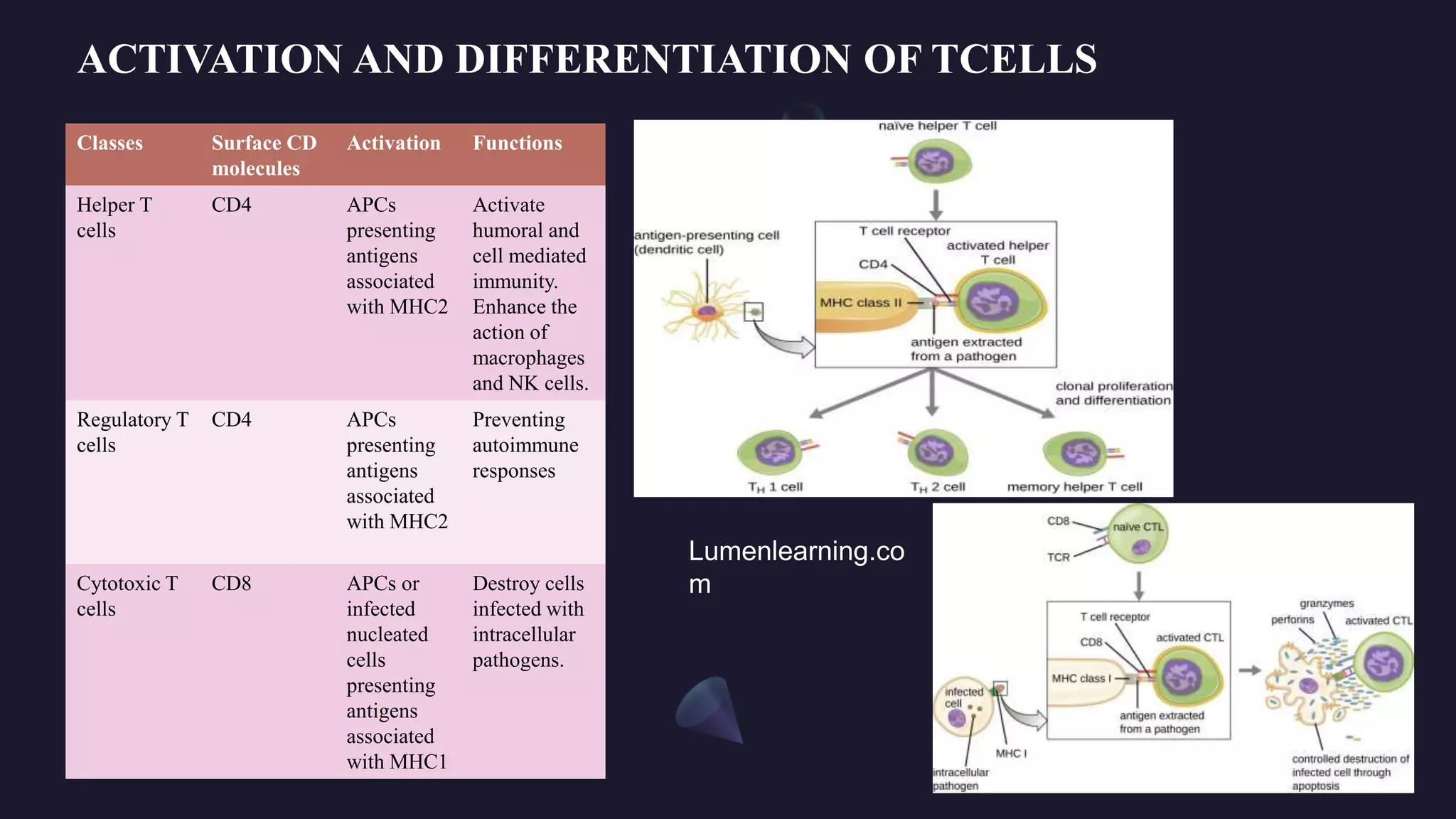
MHC1 vs MHC2: The Core Distinction That Shapes Immune Defense
MHC1 vs MHC2: The Core Distinction That Shapes Immune Defense
In the intricate battleground of human immunity, two critical players dominate: MHC class I and MHC class II molecules—separate yet intertwined components of the major histocompatibility complex (MHC) that determine how the immune system detects and responds to pathogens. While both are essential for antigen presentation, their cellular localization, molecular targeting, and functional roles differ fundamentally, influencing everything from viral surveillance to autoimmune regulation. Understanding the precise differences between MHC I and MHC II is not just academic—it’s vital for developing vaccines, designing immunotherapies, and unraveling the mechanisms behind immune-related diseases.
Cellular Distribution and Antigen Capture Pathways MHC class I molecules are ubiquitously expressed across nearly all nucleated cells, serving as constant sentinels that monitor intracellular activity. Their primary role is to display peptides derived from self-proteins and intracellular pathogens—most notably viruses—that have been processed via the cytosolic degradation pathway. Once loaded, MHC I molecules transport these antigenic complexes to the cell surface, where they are recognized by cytotoxic T cells (CD8+ T lymphocytes).
In contrast, MHC class II molecules are largely restricted to professional antigen-presenting cells (APCs)—such as dendritic cells, macrophages, and B lymphocytes—cells specialized in capturing, processing, and presenting external antigens. Unlike MHC I, MHC II achieves antigen presentation only after internalizing foreign material through phagocytosis or endocytosis. These antigens are degraded in endosomal compartments before binding to MHC II in specialized MIIC (MHC class II compartments), which are distinct from the endosomal-cytosolic sorting platforms used by MHC I.
This divergence in trafficking ensures that MHC I reflects real-time intracellular threats, while MHC II signals past or ongoing extracellular infections.
This spatial and mechanistic separation underpins their distinct immunological functions—yet their coordination is indispensable for a balanced immune response.
Structural Divergence and Peptide Binding Specificities The structural architecture of MHC I and MHC II molecules reflects their specialized antigen-processing roles. MHC I consists of a heavy alpha chain non-covalently bound to β2-microglobulin, forming a peptide-binding groove that accommodates short peptides of 8–10 amino acids.
These peptides are predominantly endogenously derived—fragments produced from cytosolic proteins that have been degraded by proteasomes and transported into the endoplasmic reticulum via TAP (transporter associated with antigen processing). The peptide binding cleft is closed at both ends, emphasizing MHC I’s preference for tightly packed, denaturated fragments optimized for CD8+ T cell recognition.
Unlike MHC I, MHC II loading occurs in acidic endosomal compartments, where invariant chain (Ii) initially blocks the peptide-binding groove to prevent premature binding; only after proteolytic cleavage is the narrow binding cavity exposed, ensuring only genuinely endogenous antigens—delivered via the JSON pathway—are presented to CD4+ T cells. This structural adaptation ensures that MHC II’s antigenic repertoire reflects external threats rather than internal cellular activity.



Related Post

Unblocked Fun Awaits: How Classroom 6X Unblocked Games Are Revolutionizing Student Playtime
Sofia Cromwell Turns Heads In Skimpy Green Bikini Photo Drop
Jeh Johnson Bio Wiki Age Parents Wife Education DHS MSNBC Biden and Net Worth

