Mastering the Art of Elimination and Substitution: A Comprehensive Guide to Sn1, Sn2, E1, E2 Reactions
Mastering the Art of Elimination and Substitution: A Comprehensive Guide to Sn1, Sn2, E1, E2 Reactions
Chemistry students, aspiring organic chemists, and researchers alike know that predicting reaction outcomes is as much an art as it is a science. At the core of organic transformations lie four fundamental mechanisms—SN1, SN2, E1, and E2—each governing substitution and elimination processes with distinct traits and conditions. Understanding their differences, conditions, and reactivity patterns empowers chemists to design efficient synthetic routes while avoiding costly missteps.
This in-depth exploration deciphers the Sn1 vs Sn2 E1 vs E2 chart—not just as separate reactions but as interconnected forces shaping molecular architecture in both laboratory and industrial settings.
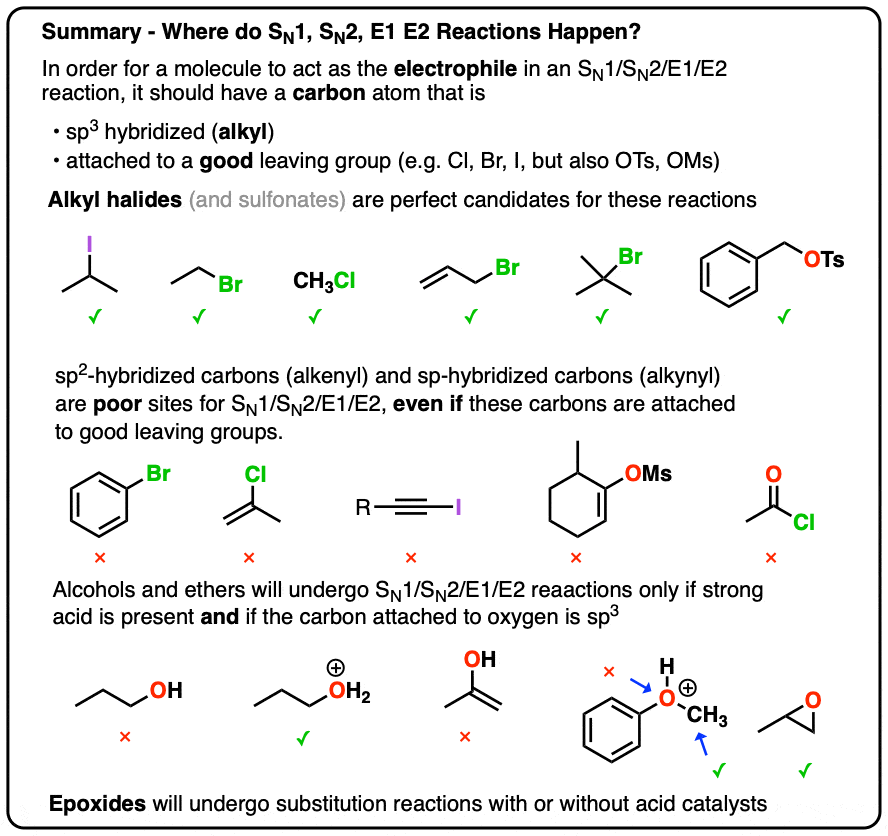
Substitution and elimination reactions lie at the heart of organic synthesis, driving the replacement of atoms or groups and the formation of double bonds. SN1 and SN2 mechanisms describe substitution pathways, where nucleophiles displace leaving groups under varying steric and electronic conditions.
Meanwhile, E1 and E2 processes govern how alkenes form through elimination, playing key roles in everything from drug development to fuel processing. By integrating these mechanisms side by side, a clear chart emerges—one that reveals similarities, contrasts, and strategic applications. This chart transcends mere categorization; it serves as a predictive tool, enabling chemists to tailor reaction conditions with precision and confidence.
Sn1 vs Sn2: Substitution Reactions Defined
The SN1 and SN2 mechanisms represent two contrasting paths for nucleophilic substitution, each defined by nucleophile strength, solvent polarity, and substrate structure.SN1 reactions proceed via a two-step process: first, the departure of the leaving group forms a carbocation intermediate; then, the nucleophile attacks from either side, resulting in a racemic mixture (if chiral). This unimolecular process favors tertiary substrates, metastable carbocations, and polar protic solvents—conditions that stabilize the charged intermediate. “The carbocation intermediate is the defining hallmark of SN1,” explains organic chemist Dr.
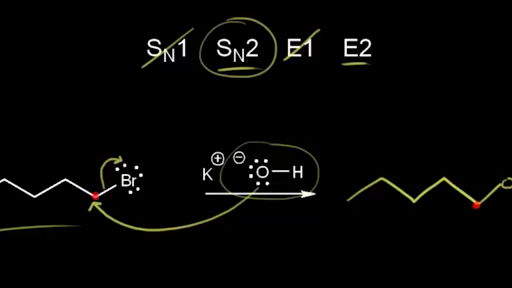
Elena Markov, “its stability dictates feasibility—tertiary substrates excel because hyperconjugation and inductive effects stabilize the positive charge.” In contrast, SN2 reactions are bimolecular and concerted—nucleophile attack occurs simultaneously as the leaving group departs—with backside displacement leading to inversion of configuration. Primary and secondary substrates dominate due to less steric hindrance. Polar aprotic solvents enhance nucleophilicity by minimizing ion solvation.
Key differences summarize the competitive nature of SN1 and SN2:
- Mechanism: SN1 is stepwise with a carbocation intermediate; SN2 is concerted with no intermediate.
- Sterics: SN2 is hindered by bulky groups; SN1 tolerates and often requires tertiary substrates.
- Rate Dependence: SN1 rate depends only on substrate; SN2 depends on both substrate and nucleophile concentration.
- Stereochemistry: SN1 produces racemates; SN2 yields inverted products.
E1 vs E2: Elimination Pathways Explored
Elimination reactions carve double bonds from saturated precursors, producing alkenes as key outputs. E1 and E2 mechanisms split along pathways distinguished by rate laws, intermediates, and stereochemical control. E1 reactions are also unimolecular, initiated by leaving group departure to form a carbocation, followed by proton abstraction to form the alkene.“The E1 mechanism favors stable carbocation formation,” notes renowned physical chemist Dr. Raj Patel. “This makes E1 particularly effective for tertiary substrates in polar protic solvents, where ion stabilization is critical.” However, E1 is generally less selective, often leading to mixtures due to carbocation rearrangements and multiple elimination pathways.
In contrast, E2 is a single-step, bimolecular process requiring strong base creation and anti-periplanar alignment between the leaving group and adjacent proton. This “concerted deprotonation and bond break” ensures stereospecific outcomes—Hofmann elimination protocols rely on this for selective alkyne formation. “E2’s strength lies in its stereochemical precision,” Patel adds.
“When base attack and elimination occur in sync, geometry governs product identity—crucial in designing regioselective syntheses.”
Critical distinctions between E1 and E2 include:
- Rate Factors: E1 rate depends solely on substrate; E2 rate requires both substrate and base concentration.
- Intermediate Lifecycles: E1 forms a carbocation intermediate; E2 avoids intermediates entirely.
- Base Influence: E2 is highly base-dependent; E1 less so.
- Steric Effects: E1 tolerates moderate steric bulk better; E2 requires accessible proton and leaving group.
Visualizing the Mechanism Landscape: A Side-by-Side Chart
A unified chart mapping SN1, SN2, E1, and E2 mechanisms clarifies their relationships, complementarities, and strategic uses. Unlike isolated boxes, this integrated diagram reveals how reaction conditions direct displacement or elimination.At the center lies nucleophilicity/stereochemical dynamics. To the left: SN1 and E1—both unimolecular, carbocation-dependent, and favored by polar protic environments that stabilize charges. Their convergence in substrates (tertiary) and solvents defines overlapping but distinct domains.
To the right, SN2 and E2—bimolecular, acceleration by strong bases, and antiperiplanar geometry—emerge as stereochemically selective alternatives. The chart’s cross-legged structure highlights cross-reactivity: tertiary substrates skew toward E1 or SN1, while primary substrates lean into E2, with intermediate geometry dictating elimination over substitution.
Beyond theory, application defines the utility of each mechanism.
SN1 substrates like tert-butyl bromide excel in forming stable carbocations for industrial esters and polymers. SN2’s inversion of configuration drives chiral synthesis and pharmaceutical intermediates. E1’s polymorphism enables complex alkene synthesis under acidic conditions, while E2’s precision underpins precision organic transformations—from cortexi reporting alkene stereochemistry to selecting specific double bonds in medicinal chemistry.
“The chart isn’t just categorization—it’s strategizing,” observes Professor Elena Cruz, organic synthesis expert. “Knowing who plays which role in which condition lets chemists preempt side reactions, optimize yields, and innovate.”
The Unified Forces of Organic Transformation
Elimination and substitution reactions are not isolated phenomena but interdependent processes shaped by molecular architecture, solvent, temperature, and reagent choice. The Sn1 vs Sn2 E1 E2 chart transcends a mere classification tool; it embodies the nuanced logic underlying modern organic chemistry.Each mechanism offers distinct advantages—SN1’s stability, E2’s precision, SN2’s speed, E1’s accessibility—making reaction selection a deliberate act of chemical foresight. As synthetic challenges grow more complex—from development of new drugs to sustainable fuel chemistry—mastery of these mechanisms becomes not just a skill, but a necessity. In mastering these pathways, chemists gain the power to sculpt molecules with intentionality, turning reaction mechanisms into blueprints for innovation.
Your understanding of substitution and elimination has never been more critical—or more powerful. Whether designing a complex molecular staircase or refining a titanic industrial process, the Sn1 vs Sn2 E1 E2 framework provides the clarity to navigate chemistry’s deepest complexities.

Related Post

Honda CR-V Sport Touring: The Cruising Powerhouse at a Reasonable Price

Embracing Freedom The Philosophy of Pure Nudism

Morristown TN Directions: Mastering Navigation Through Morristown’s Dynamic Urban Landscape

Master the Art of Covert Strike: The Warhammer Infiltrator Unveiled by The Datasheet Guide

